Review Article
Concise Review: Considerations for the Formulation, Delivery and Administration Routes of Biopharmaceuticals

Amir Mohammed Alsharabasy*
Radiation Biology Department, National Center for Radiation Research and Technology, Atomic Energy Authority, Cairo, Egypt
*Address for Correspondence: Amir Mohammed Alsharabasy, Radiation Biology Department, National Center for Radiation Research and Technology, Atomic Energy Authority, Cairo, Egypt, Email: [email protected]
Dates: Submitted: 27 May 2017; Approved: 26 June 2017; Published: 28 June 2017
How to cite this article: Alsharabasy AM. Concise Review: Considerations for the Formulation, Delivery and Administration Routes of Biopharmaceuticals. Arch Biotechnol Biomed. 2017; 1: 033-053. DOI: 10.29328/journal.abb.1001004
Copyright License: © 2017 Alsharabasy AM. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Biopharmaceutical; Drug formulation; Delivery; Administration routes
ABSTRACT
The drugs of biological origins have attracted the attention of many pharmaceutical companies where it is essential to protect the heterogeneous nature and the optimal three dimensional structures of the different macromolecules. These molecules are used in both the investigation and therapy purposes, so their maximum activities should be maintained. This requires the designing of certain delivery formulations that suits the macromolecule nature, its target organ, the required dose and delivery route, and that’s why the biotech companies invest millions of dollars towards achieving that. The first main focal point of this article includes the recent developments in the formulation technologies for several biomacromolecule classes. The second focal point concentrates on the current considerations for optimizing their delivery for a maximum performance in the body.
INTRODUCTION
Biopharmaceuticals; from a classical point of view, are defined as a subset of pharmaceuticals of inherent biological nature and manufactured using different biotechnological approaches including genetic engineering, monoclonal antibody production and vaccine bio-manufacturing [1,2]. There’s a confusion about the type of molecules classified as biopharmaceuticals and the others called drugs (pharmaceuticals of chemical origin) with diverse definitions and terms used on the level of standard regulations, science and industry. However, this article uses the classical definition of the biopharmaceuticals which encompasses variety of molecules such as nucleic acids, proteins, peptides, vaccines, and polysaccharides [3]. The different types of the bioactive macromolecules, their developmental stages and recent applications were summarized by Walsh, 2003 and Ho and Gibaldi, 2013 [4,5]. The term pharmaceutical or medicine will be used to include both the biopharmaceuticals and drugs. Moreover, the terms biological products and drugs will be used interchangeably with biopharmaceuticals as expressions only, not based on their scientific definitions. Production of the biopharmaceuticals dates back to the late 1700s with the first time of vaccine production against smallpox in cows. Currently, over 900 medicine products are developed around the world against different diseases [6]. The biopharmaceutical products represent around 13 % of these drugs, and about 70 % of them belong to the potential first-in-class products [7]. The recorded tremendous sales growth of this important class of pharmaceuticals was about 163 billion USD in 2012 [8] with invested 18.3 % of the sales in the Research and Development [9].
As macromolecules with high molecular weights, the biopharmaceutical molecules have complex structures with high sensitivity to the surrounding conditions such as humidity, medium composition and temperature. Accordingly, maintaining certain storage, transportation and disposal conditions for the final products is essential for amaintained stability and proper efficacy with eliminating the unnecessary costs and wastage under certain Current General Manufacturing Practices (CGMP) [10,11]. Handling of the different biological products as well follows certain policies which ensure cold chain compliance with effective management [12]. This requires the development of suitable formulation and delivery issues able to challenge the distinct properties of drugs, their high molecular weights, three dimensional (3D) structures, stability, and their various delivery routes for efficient activities. Various dosage forms composed of one or more bioactive macromolecules as the active ingredient(s) with number of excipients have been designed with approved effects against number of diseases. The research in the field of excipient manufacturing for improving the composition of these different forms to suite certain administration routes has attracted the attention of many researchers. These efforts concentrate on the excipient functionality within the dosage form, their safety and efficacy accompanied with the quality and processability of new forms under certain regulatory and compendial approaches. This is supported by new evidences that number of excipients can act as active ingredients, in addition to their main functions such as controlling the flowability, stability, dissolution and palatability of the active drug [13]. This review therefore summarizes the stages of the therapeutic biopharmaceutical formulation starting with the candidate drugs and the several types of associated formulations focusing on the controlling factors for their designing, and how to characterize and control their properties. Moreover, the various classes of the used excipients along with the different administration routes have been discussed.
FORMULATION OF BIOPHARMACEUTICALS
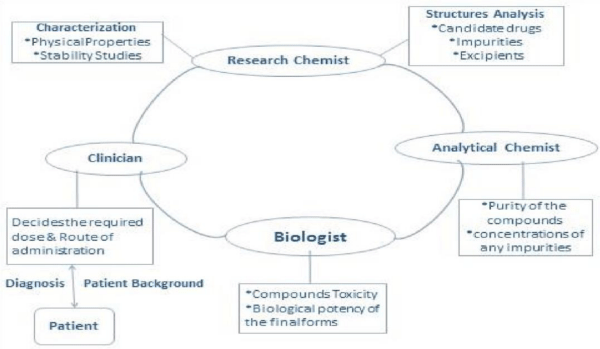
Formulation is the process by which the active drug(s) is converted into a safe, efficient and convenient form to be administrated by patient through its (their) combination with other chemical additives [14]. It involves the collaboration among the research chemists, analytical chemists, biologists and clinicians (Figure1). The background of the patient (e.g., sex, race, age) and location may also govern the formulation process and the final required form.
The process consists of two main stages: Pre-formulation stage and the formulation stage itself:
The pre-formulation stage (Formulation selection)
Once a group of compounds have been identified as efficient candidate drugs, their initial characteristics are assessed by the development scientists within a period which can last between three and six months [15]. As a pre-nomination stage, certain pre-formulation studies are performed to evaluate the structural properties, degradability, biophysical and physicochemical properties of the macromolecules [16]. Accordingly, the formulation type which can preserve the integrity and activity of the molecules with acceptable shelf-lives can be decided as the master formula. The process starts with the techniques used for analyzing the 1ry, 2ry and 3ry structures of the candidate macromolecules, with testing the types and levels of the impurities. This is followed by assessing the functionalities of the different molecules using different screening techniques, along with studying their solubility and stability for the optimum developability of the desired biological products.
Based on the close interaction among the process research, analytical and medicinal chemistry, pharmaceutical science and safety assessment, various techniques can be used to characterize both the drug substances and their final pharmaceutical products. The number of tests differs from an organization to another; some companies perform detailed characterization of the compounds, while others perform only the minimum essential number for a faster progressing into more advanced developmental stages [17,18]. Despite that, there is a continuous development in the applied technologies to analyze the different compounds that are utilized if appropriate taking into consideration the lot-to-lot variations. The different protocols and their aims are abstracted below with summarizing the associated techniques in table 1.
| Table 1: Summary of different pre-formulation and formulation characterization techniques. | ||
| Assay | Purpose | Ref. |
| CHN Elemental Microanalysis | Elemental composition | [47] |
| NMR | Structure& molecular dynamics & Product integrity | [24-27,29] |
| MS | Chemical degradants | [28,41] |
| HPLC | Quantitative& qualitative analysis | [29-41] |
| SEC | Molecular weight distribution & Product Aggregates | [35,41] |
| IR/ UV spectroscopy | Detection of concentrations | [40,41] |
| CEX | Charge variants | [30] |
| ICIEF | Charge variants & pI determination | [51,52] |
| XRD | Crystallographic analysis | [47,48] |
| DSC | Thermal/conformational stability | [48] |
| DLS | Aggregation & polydispersity | [50] |
| FTIR | Conformational stability& Polymorphism& Purity | [42,45,46] |
| CD | Conformational stability& Polymorphism | [43,45] |
Physicochemical and biophysical characterization protocols: Due to the different types of biopharmaceuticals with their complex structures and properties, a single analytical method is not sufficient to assess the required parameters of the candidate molecules and the final products. That’s why a wide range of orthogonal approaches are used to characterize the different molecules from physicochemical, biophysical, and functional points of view in precise, accurate and robust ways [19,20]. Some of these approaches belong to the quality assurance and control processes and can be used in combination with other appropriate tests for the final dosage forms such as those for the uniformity and physical properties [21]. In general, setting the acceptance criteria for the biological drug substance/ product is amenable to changing, revision and improvement with gaining more experience on the studied molecules and products. The safety and efficacy are the basic criteria by the biotech companies during these stages [22].
The essential properties of the macromolecules are characterized once synthesized or extracted through the downstream processing by the related experts. Their identity is confirmed through detailed protocols to fully understand their behaviours in the body including the absorption, plasma protein binding, solubility and clearance by the liver and kidneys [23].
Determining the composition and primary structures of each type of molecules is essential for the further formulation stages. The structure and molecular dynamics are characterized using the Nuclear Magnetic Resonance (NMR); a powerful technique that was improved to suite the analysis and identification of very small amounts of macromolecules. Moreover, it has advanced usage in process monitoring and preformulation with essential usage in testing the product integrity during the formulation process [24,25]. The usage of 2D NMR has excellent applications in polysaccharide, oligonucleotide and small proteins analysis with limitations for the larger proteins [26,27]. Furthermore, to overcome some of the Mass Spectroscopy (MS) limitations in the biopharmaceutical analysis, it’s employed coupled with NMR [28].
The High Performance Liquid Chromatography (HPLC) is a very sensitive method for detecting the drug identity [29]. It’s used in combination with Cation Exchange chromatography columns (CEX) to estimate the deamidation rates and detect the charge variants with monitoring the charge heterogeneity of the monoclonal antibodies [30]. In addition, hyphernated systems of HPLC or Liquid Chromatography with MS and/or NMR have been designed to characterize the different biomacromolecules, especially the glycosylated macromolecules [29,31].
The recombinant proteins isolated during the downstream processing often suffer from the instability leading to their aggregation and/or inactive conformation with impacts on the efficacy and immunogenicity of the protein as well as the process economics [32]. Certain robust analytical techniques are used for monitoring and quantification of the resulting aggregate contents [33]. Although these analyses are performed throughout the entire lifecycle of the production, certain requirements for each assay are needed for each developmental stage [34]. Size Exclusion Chromatography (SEC)-HPLC instruments are used for testing the Aggregation/degradation of the macromolecules with any levels of impurities [35] and they have met different developmental stages for the optimum band broadening [36-38]. The theory behind the usage of SEC, including the thermodynamics for the chromatographic separation of proteins and the kinetics and resolution of separation were summarized by [39]. Detecting the concentrations of different macromolecules, especially proteins can be achieved using UV detectors, where many Amino Acids (A.A), especially the aromatic class, give absorption for light in the UV region [40]. SEC-HPLC unit combined with U.V detector working at two different wavelengths was used for detecting and quantification of IgG1 protein including the monomer units and aggregates with a high linear range for the major species [41].
This method of detection proved its higher sensitivity than the multiangle light scattering detectors.
The routine testing of the candidate molecule structures and whether the manufacturing process or the storage conditions may cause any changes in the molecular conformation and stability depend on both the Circular Dichroism (CD) and Fourier Transform Infrared Spectroscopy (FTIR) techniques. They are also efficient in the distinguishing and quantitative analysis of the polymorphic forms of the different drug macromolecules can as well [42,43]. In general, CD is more sensitive for the α–helices of proteins, while FTIR has more sensitivity for the β-sheet secondary structures [44,45]. Moreover, FTIR defines the unique certain functional groups within the structure of each molecule and is currently used for evaluating the purity of different biopharmaceuticals and whether any solvent is included within their samples [46].
Due to the high stability and less solubility of the crystalline molecules than the amorphous analogues, the crystalline biological products are more desirable for the development [47]. The crystalline structure of the candidate macromolecules during the preformulation stage as well as that for the different products is analyzed with testing the stability of the solid forms. X-Ray powder Diffraction (XRD) is generally used for quantification of the crystallinity, but with limited sensitivity to the low degrees of the amorphous structures that can be tested using the thermal techniques (e.g., Differential Scanning Calorimetry (DSC)) [48].
Dynamic light scattering (DLS) is a sensitive technique that depends on the scattering of light by the macromolecules which fluctuates according to their sizes [49]. It’s used for estimating the extent of aggregation/degradation of particles along with the size distribution within the tested samples, so it is important in testing the physical stability of the different biomacromolecules with studying the effects of different pH values and buffers [50].
Choosing of excipients: The excipients are essentially inactive substances of natural or synthetic origin which are added to the active drug/prodrug molecules during the manufacturing process or to the final dosage form as bulking agents or fillers for different purposes [13]. They have become basic components in the different drug formulations where they may act to stabilize the macromolecule during the manufacturing, storage and handling stages [53]. For instance, some inactive ingredients are added to the protein formulations for their protection from aggregation according to certain mechanisms summarized in the report of Ohtake et al. 2011, [54]. Others control the drug release from the formulation till reaching its target organ for efficient performance and protecting the other organs with keeping their concentrations in the plasma below the toxic level [55]. Some excipients are added to improve the compliance of dosing, or to increase the patient acceptability for the drug by giving the product different colors and tastes. Others are added during the manufacturing to facilitate the powder flowability, ensure the physical stability of the final products as well protect the drug from degradation over the shelf life. Furthermore, some excipients have therapeutic effects in addition to the active ingredients such as controlling the drug availability by modulating its solubility or permeability [56], facilitating its absorption [57] or modifying the viscosity, especially with the poststerilization changes [58].
The safety information and assessment criteria of the different excipients differ from one to another based on the structure, formulation type and the administration routes [59]. Accordingly, the International Pharmaceutical Excipient Council (IPEC) have categorized them into two main classes summarized in table 2 according to Nema and Brendel, 2010 [60].
| Table 2: Different classes of excipients used in the pharmaceuticals industries. | |||
| Class | Type | Properties of the substances | Requirement |
| Class (1) Already Existing Excipients |
A | Have already established animal safety data. | More safety tests prior to using in formulations for human use. |
| B | Already used within the drug formulations by human for certain administration routes; required for other routes with different doses. | Additional safety criteria are needed to be assessed prior to using in alternative administration routes. | |
| C | Established types, but will be combined together or modified for other purposes within the formulation/administration routes. | New safety data for the resulting new structures. | |
| Class (2) New Chemical materials |
- | Firstly to be used in drug formulation. The choice should follow certain regulatory requirements which may influence the regulator y approval of the biological product. | Safety assessment according to the USP -NF Excipient Biological Safety Evaluation Guidelines [53] [64]. |
Whatever the class of excipient, the pre-clinical safety studies follows either the European or American IPEC-safety testing guidelines [61-63]. Different substances are used by the biotech companies as excipients majorly as inactive ingredients and some of them have therapeutic effects. These; alternatively, are organized based on the mode of action into the categories shown in table 3.
| Table 3: Different categories of Excipients used in the biopharmaceutical industries. | ||
| Excipient | Action | Examples (concentration: wt%) |
| Preservatives | -Used for drug products with a humidity degree and with the diluent for the lyophilized proteins. -Inhibit the microbial growth with ensured product sterility throughout both the shelf life and the duration of drug usage [65]. -Used at certain concentrations to prevent the protein aggregation, especially with drugs of multi-doses *Antioxidants: Considered preservative agents for either scavenging of the oxygen-free-radicals [66], or chelating agents for the trace metal, so protect from contaminants. |
*Amino acids: Cysteine, Methionine [67] *Vitamins: (A,E, and C) [68] Methyl paraben (0.05-0.2 %) [69,70], Benzyl alcohol (0.9 %) [71], phenol (0.15-0.5 %) [72,73], m-cresol (0.15-0.35 %) [74], EDTA (1 part preservative to 2-4 parts of EDTA) [75,76], Selenium [68] |
| Sorbents | Used as enteric coatings to protect the tablets/capsules from liquid absorption or gas adsorption. | *Natural: Waxes, Fatty acids, Plant fibers. *Synthetic plastics. |
| Fillers | -Inert and compatible substances that fill the size of the final drug product (tablet or capsule) to facilitate its production and suitable usage. -They are non-hygroscopic, cheap, safe, and don’t catalyze any side reactions or drug degradation. |
*Disaccharides: Lactose, Sucrose *Monosaccharides: glucose *Polysaccharides: Plant cellulose, Some oils *Synthetic: Ca-phosphate& carbonate Mg- stearate, alcohols (e.g., mannitol, sorbitol) |
| Buffering Agents |
-Control the pH of the drug formulation and the properties of the drug suspension on dissolving of the lyophilized formula [65]. | Imidazole, Phosphate. Succinate, Acetate, Citrate |
| Salts | -Certain types with certain concentration ranges are used to control the ionic strength of the formulation and the protein solubility, physical stability, and isotonicity as summarized by Lam et al., [77]. Some salts are added to modify the viscosity of the different formulations [78]. -Addition of some buffering salts helps to stabilize some encapsulated proteins [79,80]. |
Different salts belonging to the Hofmeister series |
| Metal ions | Certain types with certain concentrations are included in the formulation by forming coordination complexes when they are needed as cofactors for the essential actions of proteins [65] | *Zn+2 ions in insulin formulations at pH 7.4 produces the Semilente form and at pH 5.5 produces the Ultralente form [81]. *Ca+2 ions (upto 100 mM) in rhDNase formulations [82]. *Ca+2 and Sr+2 ions in Factor VIII formulations [83]. |
| Surfactants | -Amphipathic molecules to prevent any surface induced degradation due to protein adsorption or denaturation [65]. -The surface layers of these molecules can prevent the adsorption of protein molecules at their interface with vial or air [84]. |
*Fatty acid esters of sorbitan polyethoxylates Polysorbates 20, and 80. |
| Anti-adherents | -Provide non-stick surfaces to reduce the adhesion between the drug powder, the punch faces and the tablet punches during the compression step of formulation [85]. -Prevent the agglomeration of the macromolecule chains during the manufacturing process. -Decrease water vapour permeability and drug dissolution rate [86,87]. |
Talc, Mg-stearate |
| Binders | Hold the ingredients of the tablet together to give final formulae with suitable mechanical strength, especially with those of low drug loading [88,89]. |
*Disaccharides: Sucrose, Lactose. *Natural polysaccharides Starch, Cellulose, their derivatives) *Synthetic polymers: Polyvinylpyrrolidone(PVP), Polyethylene glycol (PEG) *sugar alcohols: Xylitol, Sorbitol Proteins: Gelatin |
| Coatings | -Protect the tablet ingredients from the environmental conditions (e.g., moisture, air and light). -Enhance the mechanical stability, taste and odour of the final formulation [90]. -Control the drug release and are responsible for the identification of some drug products [91]. -May be of the inner type used with tablets that will release their contents in the small intestine, not stomach [92] or external types. -Some Anti-adherents, Surfactants and Coloring agents are considered types of the coatings. |
*Outer film coatings: Cellulose acetate, Hydroxypropyl methylcellulose (HPMC), Chitosan, Proteins (e.g., Gelatin coatings for capsules). *Synthetic polymers. *Inner coatings: Phthalate, Shellac, |
| Coloring agents | -Chemical compatible agents with consistent molecular size and colours. -Improve the formulation stability and appearance to be acceptable by patients and make it easy to be identified [93,94]. -Protect the light sensitive drugs [95]. -The container for the drug itself may have certain color for keeping the drug product stable. |
-Synthetic dyes. -Natural coloring agents. -Food pigments |
| Disintegrants | Important in the dispersion of drug product on becoming wet, so help in the release of its active contents within the digestive tract [96]. | *Cross-linked polymers: PVP, Cellulose derivatives, Alginate, Modified starch. |
| Flavouring agents | -Natural or synthetic Substances to improve the acceptance of the patient for the drugs with unpleasant taste [97] by producing cooling sensation in the mouth [98]. -The type differs according to the required taste of the product (e.g., bitter, salty, sour, sweety). |
Cherry, Peach, Raspberry, Apricot, Mint, Vanilla flavours. |
| Glidants | Used during the drug product manufacturing to improve the powder flowability by reducing the frictions between the particles [99]. | Starch, Colloidal fused silica, talc, Mg-Carbonate, Glycerides. |
| Lubricants | -Substances to prevent the coherence between the different component particles and frictions with the tablet punches during the compaction. -Facilitate the tablet ejection [100] through different mechanisms [101,102]. -They may slow the disintegration of the drug product (e.g., poor hydrophilic lubricants with no glidant or anti-adherent activities). |
Figure 15.2 in chapter 15 of Tiwary et al., [103] shows the lubricant effects on drug dissolution during the preformulation studies to choose the best types. Examples: Starch, Stearic acid and its salts |
For the biological drugs of similar properties (e.g., therapeutic peptides of different numbers of A.A), the effects of the molecular size on the solubility are tested [104]. Similarly, once a group of excipients were decided, their effects on the quality of solubility are tested to evaluate whether they cause salting-in or out of the drug [105]. Assessment of the drug dissolution behaviour as a function of temperature is also performed at a certain range focusing on the physiological temperature (37oC) [106]. The thermodynamic solubility can be tested by HPLC, while the DSC and XRD are used to analyze the undissolved material to monitor any phase changes, so the solvents for crystallization can be chosen as well [107,47].
Determination of the Partition and Distribution coefficients is essential to estimate diffusivity of the drugs and membrane permeability, especially for those absorbed by passive diffusion [108]. Different methods are used based on both simple and more advanced techniques with various computer routines [18].The different materials of different crystallinity can absorb water with different degrees. The amorphous forms have characterized irregular organization of the forming atoms making structures with high surface area, so attract more water than the corresponding more crystalline forms to be more hygroscopic [109]. As a result, these molecules are more susceptible to dissolution with higher rate of degradation and less chemical stability [110]. That’s why the measurement of hygroscopicity of the candidate macromolecules within a certain time period and different temperatures is essential for testing their physical and chemical stability [111]. Accordingly, these measurements are performed in combination with the crystalline properties, thermal stability and molecular structure determination during the different stages of formulation for monitoring the overall process. The results represent important cornerstone for the designing of certain dosage formulae, especially on the transportation of the final products and the needing for certain packaging forms [50]. The choice of certain hygroscopicity degrees nevertheless differs from a drug product to another and the decision is based on the whole tests not only the hygroscopic assays as well as the degree of hygroscopicity of the added excipients. Callahan et al., 1982; Kumar et al., 2007 and Murikipudi et al., 2013 summarized the different techniques used for detecting the hygroscopicity of both the active and inactive ingredients [112-114].
Stability investigations: Many biological products, especially the native conformation of the therapeutic proteins have low thermodynamic stability with more susceptibility to change into unfolded inactive conformations accompanied with the loss of the optimal 3D architectures [115,116]. These alterations may occur under high or relatively small changes in the surrounding conditions. These include temperature, medium composition (e.g., pH, salt content and presence of oxidizing agents at certain concentrations), light, agitation and shear strain [117,118]. The purification, processing, storage and delivery stages may lead to the drug degradation. This may be chemically (e.g., oxidation, hydrolysis, deamidation, disulfide rearrangement within the macromolecular structure) or physically through its destabilization with denaturation, aggregation and insoluble particulate formation [119-121]. Details and mechanisms of the biopharmaceutics degradation are summarized in the report of Parkins and Lashmar, 2000 [51]. These changes lead to the loss of activity which may accompany immunogenic response. Accordingly, long term stability studies for both the bulk drugs and final products are performed to investigate their stability at different environmental and storage conditions, so both the shelf lives and expiry dates can be determined and improved [122]. These findings are essential for the next developmental stages for formulating a stable drug under CGMP and choosing the suitable inactive ingredients that can preserve its integrity and activity [123]. This also supports the clinical trials by guaranteeing the manufacturing of suitable products with high quality [124] and helps in the designing of proper protective packaging systems as well as in some post-marketing purposes.
In general, there is a group of real-time and accelerated stability compendial methods to test the various candidate biological drugs from physical, chemical, conformational, thermal, and photo-stability aspects taking into consideration their states of matter [125-127]. Even though there is a wide range of techniques available, the choice of specific types depends on the molecule characteristics, the required purity and potency as well as the expected type of degradants resulting from the main drug [128]. Moreover, subsets of valid methods for the characterization of drugs release can be also validated for usage in the stability studies. Examples for these protocols used in vaccines and gene-therapy products manufacturing are summarized in table 3 of Shintani report, 2013 [20]. In general, the forced degradation tests are performed prior to the stability studies to provide information on the drug degradability and share in deciding the best suitable stability protocols. In brief, different drug substance samples from different patches are tested under different experimental conditions (e.g., temperatures, pH, light intensity, humidity stress, oxygen and/or agitation stress as well as continuous freeze-thaw cycles for the frozen products). Analysis of the different degradants is used for getting information about the nature of drug degradation and its mechanism [19,129].
The accelerated stability protocols can test the candidate drug stability for a long time, compare the stability of different formulations and determine any possible degradation [130]. During the formulation development stage, the period of these studies differs among the companies based on the business size, time and the developmental pipelines sizes. The first system is the conservative approach of the large pharma companies which evaluate the stability of candidate formulations within a long period (from 6 months to 2 years) under real and accelerated conditions (max. temperature: 25oC) [131]. The best formulation of the highest purity is chosen for further manufacturing stages; however, despite the high confidence degree on choosing, it consumes long time and requires large number of samples with more personnel needs. The second system is the aggressive approach which consumes shorter period (15 days-2 months) for accelerated stability study of large number of trial formulations via statistical design of experiment under vigorous conditions suitable for the accelerated degradation [132]. This allows observing the optimum number of changes among the studied samples within a short period, followed by a longer period of stability study on the chosen formulations. This system is adopted by the small biotech companies without the time and resources required for the 1-2 year time stability study. Some problems may arise nevertheless in predicting the accelerated stability data for biological drugs, especially proteins as well as in correlating the obtained data under these conditions with those obtained after transforming to the real-time conditions [132].
The formulation stage
This stage starts after complete characterization of the properties of different substances supposed to be used as biopharmaceutical drugs with understanding their possible inactivation mechanisms during the prenomination stage along with the needs of both the clinician and patient. It involves the designing of suitable formulation scheme for certain drug products with certain safety, convenience and efficacy levels. This can be achieved with choosing the most suitable types of excipients, final forms and technologies that suite the administration route, the nature and whether the drug delivery is for intracellular or extracellular effects. The formulation technologies thus can’t be discussed separately from the different accompanied delivery routes summarized in the following section.
ADMINISTRATION ROUTES FOR THE BIOPHARMACEUTICAL PRODUCTS
Administration of the different biological products has several routes which generally begin with local delivery, and then the intracellular delivery for these drugs to perform their actions. Each route has its advantages and disadvantages based on the route itself or the used delivery technology as summarized in the report of Mitragotri et al., 2014 [133]. The injection route is the main way for the systemic delivery of the different biomacromolecules, but other routes have been borrowed as well from those used for the conventional pharmaceutical drugs such as oral, pulmonary and rectal delivery systems.
Injectable biopharmaceutical products and the applied technologies/p>
Most biopharmaceutical products are delivered via injections, where the monoclonal antibodies are generally delivered via the intravenous injection, subcutaneous delivery, or vaccines through the intramuscular route. Some formulation technologies are dependent on non-covalent interactions between the drug and its matrix (e.g., Microparticles, nanoparticles, depot formulations), but others rely on the drug modification with different methods for easier delivery approaches.
Microparticles and nanoparticles: These are used to control the sustained release of the different biological drugs (e.g., different peptides and some small molecules) and their life time in the body [134], with modulating their pharmacokinetics [135]. In brief, the drug is encapsulated within particles made of mechanically strong, biodegradable and nontoxic polymers such as poly(lactic-co-glycolic acid) (PLGA), cyclodextrins, and polyanhydrides serving as diffusion barriers [136]. Different chemical and physical methods such as electrospraying, coacervation and solvent evaporation are used in the synthesis [137,138]. In the case of nanoparticles, the carriers may be made of nano-polymeric, dendrimeric, or lipidic substances, generally used for the delivery of small biological molecules [139-141]. The release of protein from the microparticles was found to be affected by the porous structure of polymer, its molecular weight, degradation rate [133,142], the particle size, geometry, and surface properties [143] as well as the size of the loaded protein itself [144-146].
Different micro/nano particles have different applications in the adjuvant administration through conjugation to the surface of some immune cells [147], delivery of loaded certain adjuvants [147-149] and the controlled delivery of vaccines [150,151]. The development of new micro/nano carriers for peptide, protein and oligonucleotide therapeutics is under progress to overcome the problems associated with the nanoparticle-mediated delivery through pegylation modification processes [152-154].
Depot injections: This technology was designed to overcome the problems associated with the delivery of large macromolecules through injections and can be used to deliver both the small and large biological drugs. As monolithic implantable depots, the active drug is either dissolved or dispersed in a polymeric matrix, and the drug release is controlled by its rate of diffusion and the type of polymer for sustained release [155]. Different injectable in-situ forming depots are reported in the references 40-46 of Mitragotri et al., 2014 report [133].
Liquid jet injection: This technique is based on needle-free liquid injectors used against infectious diseases and for delivery of some hormones [156,157]. Some problems are associated with using this type of injection including the high cost and complexity of the method, unsuitability for the intravenous injection and the needing for personnel training as well as maintenance [158,159]. On the other hand, some trials have started for solving these problems towards more improvements for this technology [160,161].
Delivery approaches for the small biological macromolecules: The factor controlling the half-life of any drug in the body is its resistance to the hepatic and renal clearance which; subsequently, affects the frequency of drug administration [162]. The small molecules can be modified by different methods for increasing their life in the circulation before degradation. The introduction of some new molecular groups may possibly affect the drug activity, purity, polydispersity of the product with unexpected induction of the immunogenic response, nonetheless. As a consequence, new safety tests along with new formulation technologies for the resulting complex structures are required with an ability to eliminate any possible immune-response. The modification methods are as follows:Ocular delivery systems
A- Chemical modification
Depends on modifying the molecule through the covalent bonding to either a natural hydrophilic polymer (e.g., modified starch [162] and hyaluronic acid [163]), or synthetic types majorly through PEGylation [164,165] to increase the hydrodynamic radius of the drug, and thus reduce the glomerular filtration.
B- Fusion approaches
These approaches rely on the genetic fusion of the macromolecule with the Fc region of the IgG antibody or albumin. By this way, the therapeutic protein can be directly bioproduced without the need for further post-production modification [166,133].
Pumps: These are alternative devices for the delivery of injectable drugs to overcome the problems associated with any accidental injuries and needle-phobia [167]. These include the patch and implantable types for some hormone biotherapeutics such as insulin [168-170] and parathyroid hormone (PTH) [171,172]. These devices are under continuous development to overcome some problems which may arise due to the limited sterility of skin with the needing to refill more drug amount to the pump reservoir [173].
Pulmonary delivery
This route is majorly used to deliver small molecules to the lung. Many biological products are at their clinical stage of development for the delivery of therapeutic peptides with indications for cystic fibrosis, influenza virus, inflammation sarcoma, tuberculosis, and diabetes as summarized by Andrade et al., 2014 [174] in tables 7.1 and 7.2. The immunization through this route may provide excellent first barrier in the prevention of some disease, especially in the developing countries [175,176]; however, delivery of vaccines through the pulmonary route is still under the early stages of development. In spite of the success in delivery of some biopharmaceuticals, more developmental stages are required to enhance its efficacy in a safe and easy way to guarantee the optimum activity of the drug in its target tissues.
Nasal delivery route
Nasal mucosa has a high permeability and vascularization degree that enables the biopharmaceuticals to be systemically administered via the nasal route. Intranasal drug formulations for different macromolecules have been developed depending on whether the passage across the epithelium occurs via the trans-cellular or para-cellular mechanisms [177-179]. The blood flow [180], biological nature and physicochemical properties of drug [181] and possible degradation on passing through the lumen of the nasal cavity or across the nasal epithelial barrier affect the properties of these formulations as well [182,183]. Examples for the formulations delivered via this route include those for insulin delivery for diabetes treatment [184] and for vaccination [185,186].
Rectal & Vaginal administration routes
The mechanism of drug absorption from rectum differs from that in the upper part of the Gastro Intestinal Tract (GIT) only due to the physiological circumstances [187]. Unlike the monolayer of epithelia in the intestine and the lung, the vaginal tract is covered with stratified epithelia and it was found that the adaptive immunity in the vaginal mucosa; compared to other mucosal organs, is uniquely regulated [188]. Rectal immunization and intra-vaginal vaccination have been proven to be effective methods in rectal immunity and the immunity in the genital tract, respectively [189,190]. These routes of delivery anyway face some problems due to much patient inacceptability and the interruption of absorption by defecation as well.
Transdermal delivery
This route is generally used to administer low macomolecular hydrophobic drugs, where some methods are currently used to temporarily disrupt the skin for their delivery. These include certain types of small peptides [57,191], electric field and ultra-sound to deliver small biomolecules [192-193] and micro-needles for the delivery of insulin, vaccines and PTH [194]. Some biopharmaceuticals in the form of PLGAencapsulated nanoparticles within hydrogels have recently been developed for skin administration [195].
Oral delivery
Oral delivery of biological macromolecules faces some problems such as the enzymatic degradation in the GIT and the limited permeability with different absorption pathways through the intestinal epithelium which lead to their poor absorption and limited bioavailability [196]. Some techniques are now under development to improve the properties of drug formulation for oral administration and some additives are added to enhance the properties of the biopharmaceutical drugs for reaching the desired effects (Table 3). These include the usage of mucoahesive devices to deliver some hormones [197-198] and targeted nanoparticles for monoclonal antibody delivery. The surface of these particles is positively charged to bind with the negatively charged intestinal mucosa and easily permeate through it [199-200]. Variables such as the compressional force or granulation process in the production of biopharmaceutical tablets can significantly affect the drug bioavailability as well [201].
Local drug delivery
Despite the wide range of research regarding the different technologies for the systemic delivery of the biopharmaceuticals, the local delivery of some drugs such as the growth factors is the most suitable way for guaranteed targeting of specific tissues with optimum efficiency.
Ocular delivery systems: Periocular delivery is one of the administration routes for drugs to the subconjunctival, retrobulbar, and peribulbar locations using either implantation or injection techniques, but it may cause some complications such as hemorrhages in these locations nevertheless [202,203]. The intravitreal administration method uses the same techniques to directly deliver the biopharmaceutial molecule to the vitreous and retina. In spite of that, the patients are at higher risk due to the possible hemorrages, cataract occurrence, and retinal detachment [204]. Ocular formulations in the form of nanoparticles (e.g., PLGA) have been recently designed with proved ex vivo corneal permeation and in vivo anti-inflammatory effects [205].
Periodontal regeneration:Limited number of trials are now under progress for peridodontal gene therapy through the local delivery of nano/microparticles [206,207].
Intracellular delivery
After the systemic or local delivery of the macromolecule, its potential targets are mainly intracellular compartments including the mitochondrial, nuclear, and cytoplasmic molecules (e.g., enzymes, receptors, proteins, oligonucleotides) [208,209]. To achieve the internalization of the macromolecules and increase the therapeutic index, different approaches have been designed. This aims to increase the permeability of the cellular membranes, activate the fusion between the drug and protein [210] and enhance the penetration to inside the cell through the usage of nanoparticles with special sizes [211]. In general, it was found out that the choosing of a certain apprach depends on the type of the drug macromolecule [133,212].
SUMMARY
The Formulation technologies and delivery routes of the different biopharmaceuticals for different diseases can’t be studied separately. The chemical and physical properties of the drug, its biological activity, excipient types, and the administration routes should be essentially investigated as background information towards the designing of an efficient drug formulation. This is important for maintaining the maximum activity of the drug for performing its function in its target tissue. This industrial field of manufacturing has met many improvements; however, it still faces some challenges which require further research and development.
REFERENCES
- Rader RA. BioExecutive Intl. 2005; 60-65.
- Rader RA. Biopharmaceutical Products in the US and European Markets. 6th ed., 2007; 2. Ref.: https://goo.gl/f9P2o9
- Rader RA. (Re) defining biopharmaceutical. Nature Biotechnol. 2008; 26: 743-751. Ref.: https://goo.gl/Ej6u1w
- Walsh G. Biopharmaceuticals biochemistry and biotechnology, 2nd Edition. John Wiley & Sons, Ltd, Chichester, U.K. 2003.
- Ho RJ, Gibaldi M. Biotechnology and Biopharmaceuticals: Transforming Proteins and Genes into Drugs. 2nd Edition. John Wiley & Sons, Ltd, Chichester, U.K. 2013.
- Pharmaceutical Research and Manufacturers of America. Medicines in Development -Biologics (2013 report). PhRMA [online]. 2016.
- Pharmaceutical Research and Manufacturers of America. Medicines in Development-Biologics (2015 report). PhRMA [online]. 2016.
- CSDD. Biotech products in big pharma clinical pipelines have grown dramatically. Tufts CSDD Impact Report. 2013; 15: 1-4.
- PhRMA ChartPack (2015). Biopharmaceuticals in Prospective. 2016.
- FDA. Guidance for Industry Contract Manufacturing Arrangements for Drugs: Quality Agreements. 2013.
- Guidance for Clinical Investigators, Sponsors, and IRBs Investigational New Drug Applications (INDs)-Determining Whether Human Research Studies Can Be Conducted Without an IND. 2013.
- National Patient Safety Agency: National Reporting and Learning Service (2010) Vaccine cold storage. 2016.
- Lokesh B, et al. Excipients: Background/Introduction". In: Ashok K and Mahesh C (eds). Excipient Development for Pharmaceutical, Biotechnology, and Drug Delivery Systems. Informa Healthcare, New York. 2006.
- Hassan BA. Overview on Pharmaceutical Formulation and Drug Design. Pharmaceut Anal Acta. 3:10. 2012.
- Venkatesh S, Lipper RA. Role of the development scientist in compound lead selection and optimization. J Pharm Sci. 2000; 89: 145-154. Ref.: https://goo.gl/zBxer4
- Simler R, Walsh G, Mattaliano RJ, N Guziewicz, Perez-Ramirez B. Maximizing data collection and analysis during preformulation of biotherapeutic proteins. BioProcess Int. 2008; 4: 38-45.
- Li AP. A comprehensive approach for drug safety assessment. Chem Biol Interact. 2004; 150: 27-33. Ref.: https://goo.gl/VeqWjw
- Steele G, Austin T. Chapter (3): Preformulation Investigations using Small Amounts of Compound as an Aid to Candidate Drug Selection and Early Development. In: Gibson M. Pharmaceutical Preformulation and Formulation. Informa Healthcare. 2009; New York. 17-128.
- Wei Z, Emily Shacter, Mark Schenerman, John Dougherty, Lorna McLeod D. CMC Strategy Forum Report: The Role of Higher-Order Structure in Defining Biopharmaceutical Quality. BioProcess Int. 2011; 6: 54-65. Ref.: https://goo.gl/b9rkh1
- Shintani H. Development of test method for pharmaceutical and biopharmaceutical products. Pharm Anal Acta. 2013; 4: 1-14. Ref.: https://goo.gl/5RKrNA
- ICH Topic Q6B. Specifications: Test Procedures and Acceptance Criteria for Biotechnological/ Biological Products. CPMP/ICH/365/96. 1999.
- ICH Topic Q6A. Specifications: Test Procedures and Acceptance Criteria for New Drug Substances and New Drug Products: Chemical Substances. CPMP/ICH/367/96. 2000.
- ICH papers: ICH topic Q5E. Harmonised tripartite guideline: comparability of biotechnological/ biological products subject to changes in their manufacturing process. 2004.
- Berkowitz SA, Engen JR, Jeffrey RM, Graham BJ. Analytical tools for characterizing biopharmaceuticals and the implications for biosimilars. Nat Rev Drug Discov. 2012; 11: 527-40. Ref.: https://goo.gl/3gMuzv
- Wishart DS. Characterization of biopharmaceuticals by NMR spectroscopy. Trends Anal Chem. 2013; 48: 96-111. Ref.: https://goo.gl/FVUbJT
- Zuperl S, P Pristovšek, V Menart, Porekar GV, Novic M. Chemometric approach in quantification of structural identity/similarity of proteins in biopharmaceuticals. J Chem Inf Model. 2007; 47: 737-743. Ref.: https://goo.gl/tKyYNy
- Aubin Y, G Gingras, S Sauvé. Assessment of the three-dimensional structure of recombinant protein therapeutics by NMR fingerprinting: demonstration on recombinant human granulocyte macrophagecolony stimulation factor. Anal Chem. 2008; 80: 2623-2627. Ref.: https://goo.gl/G6bGfB
- Lin Y, Schiavo S, Orjala J, Vouros P, Kautz R. Microscale LC-MS-NMR platform applied to the identification of active cyanobacterial metabolites. Anal Chem. 2008; 80: 8045-8054. Ref.: https://goo.gl/xf2kqW
- Nikolin B, Belma I, Medanhodzić-Vuk S, Soberet M.High perfomance liquid chromatography in pharmaceutical analyses. Bosn J Basic Med Sci. 2004; 4: 5-9. Ref.: https://goo.gl/55pxzd
- Lim A, Barnes CS. Chapter 11: Utilization of Mass Spectrometry for the Structural Characterization of Biopharmaceutical Protein Products. In: Gross ML. et al (eds). Protein and peptide mass spectrometry in drug discovery. John Wiley & Sons, Inc.: New Jersy. 2012; 304.
- Higel F, Demelbauer U, Andreas S, Wolfgang F, Fritz S. Reversed-phase liquid-chromatographic mass spectrometric N-glycan analysis of biopharmaceuticals. Anal Bioanal Chem. 2013; 405: 2481-2493. Ref.: https://goo.gl/K3onwc
- Rosenberg AS. Effects of Protein Aggregates: An Immunologic Perspective. AAPS J. 2006; 8: 501-507. Ref.: https://goo.gl/L69suP
- Lloyd L. Size-exclusion chromatography of protein aggregation in biopharmaceutical development and production. 2014; 32: 30-35.
- Engelsman J, Garidel P, Smulders R, Koll H, Smith B, et al. Strategies for the assessment of protein aggregates in pharmaceutical biotech product development. Pharm Res. 2011; 28: 920-33. Ref.: https://goo.gl/oJKcye
- McGrath BM. Chapter (11): Factor IX (Protease Zymogen). In: McGrath BM, Walsh G. Directory of therapeutic enzymes. CRC Press Taylor & Francis Group: New York. 2006; 225.
- Popovici ST, Kok WT, Schoenmakers PJ. Band broadening in size-exclusion chromatography of polydisperse samples. J Chromatogr A. 2004; 1060: 237-252. Ref.: https://goo.gl/2yYvUv
- Ziegler A, Zaia J. Size-exclusion chromatography of heparin oligosaccharides at high and low pressure. J Chromatogr B Analyt Technol Biomed Life Sci. 2006; 837: 76-86. Ref.: https://goo.gl/G9aUmb
- Gritti F, Farkas T, Heng J, Guiochon G. On the relationship between band broadening and the particle-size distribution of the packing material in liquid chromatography: theory and practice. J Chromatogr A. 2011; 1218: 8209-8221. Ref.: https://goo.gl/KCwXGh
- Hong P, Koza S, Bouvier ES. Size-exclusion chromatography for the analysis of protein biotherapeutics and their aggregates. J Liq Chromatogr Rel Technol. 2012; 35: 2923-2950. Ref.: https://goo.gl/sifx5d
- Aitken A, Learmonth M. Protein Determination by uv Absorption. In: Walker JM (ed). The Protein Protocols Handbook. Humana Press: New Jersy. 1996; 3-6.
- Bond MD, Mark EP, Zhang Z, Wang D, Mehndiratta, et al. Evaluation of a dual-wavelength size exclusion HPLC method with improved sensitivity to detect protein aggregates and its use to better characterize degradation pathways of an IgG1 monoclonal antibody. J Pharm Sci. 2010; 99: 2582-2597. Ref.: https://goo.gl/AeFJCR
- Kipouros K, Kachrimanis K, Nikolakakis I, Tserki V, Malamataris S. Simultaneous quantification of carbamazepine crystal forms in ternary mixtures (I, III, and IV) by diffuse reflectance FTIR spectroscopy (DRIFTS) and multivariate calibration. J Pharm Sci. 2006; 95: 2419-2431. Ref.: https://goo.gl/JSHGfV
- Li CH, Xichdao N, Linda N, Chemmalil L, Edward T. Applications of circular dichroism (CD) for structural analysis of proteins: qualification of near-and far-UV CD for protein higher order structural analysis. J Pharm Sci. 2011; 100: 4642-4654. Ref.: https://goo.gl/Rkrszu
- Dalal S, Balasubramanian S, Lynne Regan. Transmuting α-helices and β-sheets. Folding Design. 1997; 2: 71-79. Ref.: https://goo.gl/H74oG3
- Dong A, James M, Mark CM, John FC. Intermolecular beta-sheet results from trifluoroethanol-induced nonnative alpha-helical structure in beta-sheet predominant proteins: infrared and circular dichroism spectroscopic study. Arch Biochem Biophys. 1998; 355: 275-281. Ref.: https://goo.gl/59Y6aF
- Li G, Gianni T, Wendy J, Zai-qing W. Applications of FTIR in identification of foreign materials for biopharmaceutical clinical manufacturing. Vibrational Spectroscopy. 2009; 50: 152-159. Ref.: https://goo.gl/xKtE71
- Yazdanian M. Overview of determination of biopharmaceutical properties for development candidate selection. Curr Protoc Pharmacol. 2013. Ref.: https://goo.gl/Pa4x2V
- Lechuga-Ballesteros D. Chapter (7): Thermal Analysis of Lyophilized Pharmaceutical Peptide and Protein Formulations. In: Costantino HR, Pikal MJ (eds). Lyophilization of Biopharmaceuticals. Springer Science & Business Media: Arlington. 2005; 283.
- Chu B. Laser Light Scattering. Annu Rev Phys Chem. 1970; 21: 145-174.
- Niazi SK. Handbook of Preformulation: Chemical, Biological, and Botanical Informa Healthcare: USA. 2007.
- Sosic Z, Damian H, Blum A, Carlage T, Lyubarskaya Y. Application of Imaging Capillary IEF for Characterization and Quantitative Analysis of Recombinant Protein Charge Heterogeneity. Electrophor. 2008; 29: 4368-4376. Ref.: https://goo.gl/A2STiS
- Michels DA, Oscar Salas-Solano, Chantal Felten. Imaged Capillary Isoelectric Focusing for Charge-Variant Analysis of Biopharmaceuticals. BioProcess Int. 2011; 9: 48-54. Ref.: https://goo.gl/pxayRq
- Parkins DA, Lashmar UT. The formulation of biopharmaceutical products. PSTT. 2000; 3: 129-137. Ref.: https://goo.gl/nFrpWQ
- Ohtake S, Yoshiko Kita, Tsutomu Arakawa. Interactions of formulation excipients with proteins in solution and in the dried state. Adv Drug Deliv Rev. 2011; 63: 1053-1073. Ref.: https://goo.gl/3dXjrb
- Kleinman MH, Lee B. Chapter (14): challenges in early formulation: turning drug substance into drug product. In: Abdel-Magid AF, Caron S (eds). Fundamentals of early clinical drug development. Wiley: New Jersey. 2006; 272.
- Mills S. Training Workshop on Pharmaceutical Development with focus on Paediatric Formulations. Archived from the original WHO. 2012.
- Hsu T, Mitragotri S. Delivery of siRNA and other macromolecules into skin and cells using a peptide enhancer. Proc Natl Acad Sci. 2011; 108: 15816-15821. Ref.: https://goo.gl/f86PXV
- Fairand BP, Razem D. Chapter (12): Radiation sterilization. In: Nema S, Ludwig JD (eds). Pharmaceutical dosage forms: parenteral medications (3rd edition, vol. 2)-facility design, sterilization and processing. Informa: London. 2010; 292.
- IPEC. Excipients in pharmaceutical dosage forms: The challenge of the 21st century. IPEC, Nice. 1998.
- Nema S, Brendel RJ. Chapter (7): Excipients for parenteral dosage forms: regulatory considerations and controls. In: Nema S, Ludwig JD (eds). Pharmaceutical Dosage Forms: Parenteral Medications, Vol. 3: Regulations, Validation and the Future. Informa Healthcare, London. 2010; 123.
- De Jong HJ. The safety of pharmaceutical excipients. Therapie. 1999; 54: 11-14. Ref.: https://goo.gl/yc2Nhc
- Steinberg M, Borzelleca JF, Enters EK, Kinoshita FK, Loper A, et al. A new approach to the safety assessment of pharmaceutical excipiens. The safety committee of the international pharmaceutical excipient council. Regul Toxicol Pharmacol. 1996; 24: 149-154. Ref.: https://goo.gl/AAPVeY
- Elder DP, Kuentz M, Holm R. Pharmaceutical excipients -quality, regulatory and biopharmaceutical considerations. Eur J Pharm Sci. 2016. 87: 88-99. Ref.: https://goo.gl/4suJ2t
- USP-NF General Chapter (1074) Excipient Biological Safety Evaluation Guidelines. 2016.
- Gokarn YR. Chapter (17): Excipients for Protein Drugs. In: Ashok K, Mahesh C. Excipient Development for Pharmaceutical, Biotechnology, and Drug Delivery Systems. Informa Healthcare, New York. 2006.
- Cacace MG, Landau EM, Ramsden JJ. The Hofmeister series: salt and solvent effects on interfacial phenomena. Q Rev Biophys. 1997; 30: 241-277. Ref.: https://goo.gl/ivDsTa
- Kamerzell TJ, Esfandiary R, Joshi SB, Middaugh CR, Volkin, DB. Protein-excipient interactions: mechanisms and biophysical characterization applied to protein formulation development. Adv Drug Deliv Rev. 2011; 63: 1118-1159. Ref.: https://goo.gl/Mn1qYG
- Ref.: https://goo.gl/HEp7u2
- Aalto TR, Firman MC, Rigler NE. p-hydroxybenzoic acid esters as preservatives. I. Uses, antibacterial and antifungal studies, properties and determination. J Am Pharm Assoc Sci Ed. 1953; 42: 449-457. Ref.: https://goo.gl/QjMwQa
- Gerbino PP. Remington: The Science and practice of pharmacy, 21st edition. Lippincott Williams & Wilkins, Philadelphia.
- Roy S, Jung R, Kerwin BA, Randolphn TW. Effects of benzyl alcohol on aggregation of recombinant human interleukin-1receptor antagonist in reconstituted lyophilized formulations. J Pharm Sci. 2005; 94: 382-396. Ref.: https://goo.gl/vJrLx7
- Christensen PA. The stability of refined antivenin. Toxicon. 1975; 13: 75-77. Ref.: https://goo.gl/6eg2FX
- Rojas G, Jimenez JM, Gutiérrez JM. Caprylic acid fractionation of hyperimmune horse plasma: description of a simple procedure for antivenom production. Toxicon. 1994; 32: 351-363. Ref.: https://goo.gl/tYXPag
- Abd-Elsalam MA, Abdoon N, Al-Ahaidib MS. What is the optimum concentration of mcresol in antivenoms? J Venom Anim Toxins incl Trop Dis. 2011; 17: 12-22. Ref.: https://goo.gl/AAD3D6
- Izzat IN, Bennett EO. Effect of varying concentrations of EDTA on the antimicrobial properties of cutting fluid preservatives. Microbios. 1979; 26: 37-44. Ref.: https://goo.gl/JfF5it
- Whalley G. Preservative Properties of EDTA, Manuf. Chem. 1991; 62: 22-23.
- Lam XM, Yang JY, Cleland JL. Antioxidants for prevention of methionine oxidation in recombinant monoclonal antibody HER2. J Pharm Sci. 1997; 86: 1250-1255. Ref.: https://goo.gl/FJR9Wy
- Liu J, Nguyen MD, Andya JD, Shire SJ. Reversible self-association increases the viscosity of a concentrated monoclonal antibody in aqueous solution. J Pharm Sci. 2005; 94: 1928-1940. Ref.: https://goo.gl/s2coMw
- Zhu G, Mallery SR, Schwendeman SP. Stabilization of proteins encapsulated in injectable poly(lactide-co-glycolide). Nat Biotechnol. 2000; 18: 52-57. Ref.: https://goo.gl/Ao2iXY
- Kang J, Schwendeman SP. Comparison of the effects of Mg(OH)2 and sucrose on the stability of bovine serum albumin encapsulated in injectable poly(D,L-lactide-co-glycolide) implants. Biomaterials. 2002; 23: 239-245. Ref.: https://goo.gl/2KUHtG
- Gualandi-Signorini AM, Giorgi G. Insulin formulations--a review. Eur Rev Med Pharmacol Sci. 2001; 5: 73-83. Ref.: https://goo.gl/1WTYoQ
- Chen B, Costantino HR, Liu J, Hsu CC, Shire SJ. Influence of calcium ions on the structure and stability of recombinant human deoxyribonuclease I in the aqueous and lyophilized states. J PharmSci. 1999; 88: 477-482. Ref.: https://goo.gl/AxQVt7
- Angelica Fatouros, Thomas Österberg, Marianne Mikaelsson. Recombinant factor VIII SQ-influence of oxygen, metal ions, pH and ionic strength on its stability in aqueous solution. Int J Pharm. 1997; 155: 121-131. Ref.: https://goo.gl/LTQKfG
- Treuheit MJ, Kosky AA, Brems DN. Inverse relationship of protein concentration and aggregation. Pharm Res. 2002; 19: 511-516. Ref.: https://goo.gl/XxFKuS
- Shah NH, Stiel D, Weiss M, Infeld MH, Malick AW. Evaluation of two new tablet lubricants-sodium stearyl fumarate and glyceryl behenate. Measurement of physical parameters (compaction, ejection and residual forces) in the tabletting process and effect of the dissolution rate. Drug Dev Ind Pharm. 1986; 12: 1329-1346. Ref.: https://goo.gl/3QPptK
- Fassihi RA, Mcphillips AM, Uraizee SA, Sakr AM. Potential use of magnesium stearate and talc as dissolution retardants in the development of controlled drug delivery systems. Pharm Ind. 1994; 56: 579-583. Ref.: https://goo.gl/x7yaBD
- Maejima T, McGinity JW. Influence of film additives on stabilizing drug release rates from pellets coated with acrylic polymers. Pharm Dev Technol. 2001; 6: 211-221. Ref.: https://goo.gl/vfvYEC
- Sugimoto M, Matsubara K, Koida Y, Kobayashi M. The preparation of rapidly disintegrating tablets in the mouth. Pharm Dev Technol. 2001; 6: 487-493. Ref.: https://goo.gl/Xk8vKx
- Shamblin S. Chapter(9): Controlled release using bilayer osmotic tablet technology: reducing theory to practice. In: Wen H, Park K (eds) Oral controlled release formulation design and drug delivery. Wiley & Sons, Inc.: New Jersey. 2010; 138.
- Siepmann J. Process and formulation factors affecting drug release from pellets coated with ethylcellulose pseudolatex aquacoat. In: McGinity JW, Felton LA (eds). Aqueous Polymeric Coatings for Pharmaceutical Dosage Forms, 3rd edition, New York: Informa Healthcare. 2008; 203-236.
- Chen S, Cao Y, Ferguson LR, Shu Q, Garg S. Evaluation of mucoadhesive coatings of chitosan and thiolated chitosan for the colonic delivery of microencapsulated probiotic bacteria. J Microencapsul. 2013; 30: 103-115. Ref.: https://goo.gl/AK3Vhb
- Dulin W. Oral targeted drug delivery systems: enteric coating. Oral controlled release formulation design and drug delivery. 2010; 213.
- Felton LA, McGinity JW. Influence of insoluble excipients on film coating systems. Drug Dev Ind Pharm. 2002; 28: 225-243. Ref.: https://goo.gl/vFzS7g
- Rowe RC, Paul Sheskey J, Owen SC. Handbook of Pharmaceutical Excipients. 5th edition, Pharmaceutical Press. 2006.
- Felton LA, McGinity JW. Influence of pigment concentration and particle size on adhesion of an acrylic resin copolymer to tablet compacts. Drug Dev Ind Pharm. 1999; 25: 599-606. Ref.: https://goo.gl/5qpTxx
- Shanraw R, Mitrevej A, Shah M. A new era of tablet disintegrants. Pham Technol. 1980; 4: 48-57.
- Chang RK, Xiaodi Guo, Burnside BA, Couch RA. Fast-dissolving tablets. Pharm Technol. 2000; 24: 52-58. Ref.: https://goo.gl/9kVxCq
- Elshattawy HH, Dane Kildsig O, Garnet Peck E. Aspartame-mannitol resolidified fused mixture: characterization studies by differential scanning calorimetry, thermomicroscopy, photomicrography and X-ray diffractometry. Drug Dev Ind Pharm. 1984: 10: 1-17. Ref.: https://goo.gl/S8WbaC
- Lin YA. Enteric-coated pellet formulation and process scale-up improvement using mono- and diglycerides as a glidant. Poster Presentation. AAPS Annual Meeting, San Diego, CA. 2007.
- Abhijit Sonje, Arun Yadav, Chandra A, Jain DA. Formulation and evaluation of immediate release tablet of antihypertensive drugs according to BCS system. Int J Therap Appl. 2012; 7: 18-24. Ref.: https://goo.gl/bm23bc
- Jones TM. Symposium on Powders. Dublin: Society of Cosmetic Chemists of Great Britain. 1969.
- Peleg M, Mannheim CH. Effect of conditioners on the flow properties of powdered sucrose. Powder Technol. 1973; 7: 45-50. Ref.: https://goo.gl/BRvPeb
- Tiwary AK. Dissolution. In: Gad SC. Preclinical Development handbook ADME and Biopharmaceutical Properties. John Wiley & Sons. Inc: New Jersey. 2008; 494.
- James KC. Solubility and Related Phenomena. Mercel Dekker Inc. 1986.
- Bai JPF, Guo JH, Mahesh VC. Use of nonactive pharmaceutical excipients in oral drug formulations: Biopharmaceutical classification system considerations. In: Katdare A, Chaubal MV. (eds) Excipient development for pharmaceutical, biotechnology, and drug delivery. Informa Healthcare USA, Inc.: New York. 182. 2006.
- Ungell A, Abrahamsson B. Chapter(4): Biopharmaceutical support in candidate drug selection. 2nd edition. In: Gibson M. Pharmaceutical Preformulation and Formulation. Informa Healthcare. 2009.
- Alsenz J, Kansy M. High throughput solubility measurement in drug discovery and development. Adv Drug Deliv Rev. 2007; 59: 546-567. Ref.: https://goo.gl/GGPNDQ
- Valvani SC. Chapter (2): The Pharmaceutical Background. In: Lee C, et al. (eds) Clinical Trials of Drugs and Biopharmaceuticals. CRC Press Taylor & Francis Group: Boca Raton. 2006; 17.
- Ahlneck C, Zografi G. The molecular basis of moisture effects on the physical and chemical stability of drugs in the solid state. Int J Pharm. 1990; 62: 87-95. Ref.: https://goo.gl/V8ZX4V
- USP, The United States Pharmacopeia. XXIII Revision, United States Pharmacopeial Convention, Rockville, Md. 1995.
- Hancock BC, Dalton CR. The effect of temperature on water vapor sorption by some amorphous pharmaceutical sugars. Pharm Dev Techn. 1999; 4: 125-131. Ref.: https://goo.gl/Ut46Zo
- Callahan J, Cleary GW, Elefant M, Kaplan G, Kensler T, et al. Equilibrium moisture content of pharmaceutical excipients. Drug Dev Ind Pharm; 1982; 8: 355-369. Ref.: https://goo.gl/tTP5XE
- Kumar L, Amin A, Bansal AK. An overview of automated systems relevant in pharmaceutical salt screening. Drug Discov Today. 2007; 12: 1046-1053. Ref.: https://goo.gl/M61VjW
- Murikipudi V, Gupta P, Sihorkar V. Efficient throughput method for hygroscopicity classification of active and inactive pharmaceutical ingredients by water vapor sorption analysis. Pharm Dev Technol. 2013; 18: 348-358. Ref.: https://goo.gl/rhBXHv
- Jaenicke R. Protein folding: local structures, domains, subunits, and assemblies. Biochemistry. 1991; 30: 3147-3161. Ref.: https://goo.gl/n8DdE8
- Pace CN, Shirley BA, McNutt M, Gajiwala K. Forces contributing to the conformational stability of proteins. FASEB J. 1996; 10: 75-83. Ref.: https://goo.gl/3n6HV4
- Wang W. Instability, stabilization, and formulation of liquid protein pharmaceuticals. Int J Pharm. 1999; 185: 129-188. Ref.: https://goo.gl/nQyt1q
- Cromwell ME, Hilario E, Jacobson F. Protein aggregation and bioprocessing. AAPS J. 2006; 8: 572-579. Ref.: https://goo.gl/Q1k1Da
- Brorson K, Phillips J. Defining your product profile and maintaining control over it, Part 4. Product-Related Impurities: Tackling Aggregates. Bioprocess Int. 2005; 3: 50-54. Ref.: https://goo.gl/CH6J7r
- Rosenberg AS. Effects of protein aggregates: an immunologic perspective. AAPS J. 2006; 8: 501-507. Ref.: https://goo.gl/qFtqqv
- Smales CM, Pepper DS, James DC. Protein modification during anti-viral heat-treatment bioprocessing of factor VIII concentrates, factor IX concentrates, and model proteins in the presence of sucrose. Biotechnol Bioeng. 2002; 77: 37-48. Ref.: https://goo.gl/fDPdCv
- McEntire J. Biotechnology Product Validation Part 5: Selection and Validation of Analytical Techniques. BioPharm. 1994; 7: 68-79.
- USP Guideline for Submitting Requests for Revision to USP-NF: V3.1 EXCIPIENTS. U S. PHARMACOPEIA. 2007. Ref.: https://goo.gl/8vDcph
- Qualification of excipients for use in pharmaceuticals. IPEC. 2008. Ref.: https://goo.gl/nN6KzX
- International Conference on Harmonisation- Quality of biotechnological products: ICH Q5C: Stability testing of biotechnological/biological products. 1995.
- FDA. "FDA Guidance for Industry PAT-A Framework for Innovative Pharmaceutical Development, Manufacturing, and Quality Assurance," September. 2004.
- WHO Annex 2. Stability testing of active pharmaceutical ingredients and finished pharmaceutical products. WHO Technical Report Series. No. 953. 2009.
- Patel J, Nadine Ritter M, Ruchi Kothari, Rashbehari Tunga, Binita Tunga S. Stability Considerations for Biopharmaceuticals: Overview of Protein and Peptide Degradation Pathways. BioProcess International. 2011; 9: 2-11. Ref.: https://goo.gl/bNSsrx
- Yu J. Intentionally Degrading Protein Pharmaceuticals to Validate Stability-Indicating Analytical Methods. BioPharm. 2000; 13: 46-52.
- Waterman KC, Adami RC. Accelerated ageing: prediction of chemical stability of pharmaceuticals. Int J Pharm. 2005; 293: 101-125. Ref.: https://goo.gl/rNzzoj
- Nieminen O, Kurki P, Nordström K. Differences in product information of biopharmaceuticals in the EU and the USA: implications for product development. Eur J Pharm Biopharm. 2005; 60: 319-326. Ref.: https://goo.gl/KxU8D6
- Kelly T. Accelerated Stability During Formulation Development of Early Stage Protein Therapeutics - Pros and Cons of Contrasting Approaches. KBI Biopharma. IBC Formulation Strategies for Protein Therapeutics. 2008.
- Mitragotri S, Burke PA, Langer R. Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies. Nat Rev Drug Discov. 2014; 13: 655-672. Ref.: https://goo.gl/g1EzCw
- Silva AC, Lopes CM, Lobo JM, Amaral MH. Delivery Systems for Biopharmaceuticals. Part I: Nanoparticles and Microparticles. Curr Pharm Biotechnol. 2015; 16: 940-954. Ref.: https://goo.gl/GG3oQa
- Vilos C, Velasquez LA. Therapeutic Strategies Based on Polymeric Microparticles. J Biomed Biotechnol. 2012; 2012: 1-9. Ref.: https://goo.gl/vrpTgz
- Reis CP, Damgé C. Nanotechnology as a promising strategy for alternative routes of insulin delivery. Methods Enzymol. 2012; 508: 271-294. Ref.: https://goo.gl/8mztFS
- Umer H, H Nigam, AM Tamboli, MSM Nainar. Microencapsulation: Process, Techniques and Applications. International Journal of Research in Pharmaceutical and Biomedical Sciences. 2011; 2: 474-481.
- Bock N, Dargaville TR, Woodruff MA. Controlling microencapsulation and release of micronized proteins using poly(ethylene glycol) and electrospraying. Eur J Pharm Biopharm. 2014; 87: 366-377. Ref.: https://goo.gl/zLAQ4d
- Shi J, Xiao Z, Votruba AR, Vilos C, Farokhzad OC. Differentially charged hollow core/shell lipid-polymer-lipid hybrid nanoparticles for small interfering RNA delivery. Angew Chem Int Ed Engl. 2011; 50: 7027-7031. Ref.: https://goo.gl/2AR7kY
- Rahman MA, Amin AR, Wang X, Zuckerman JE, Choi CH, et al. Systemic delivery of siRNA nanoparticles targeting RRM2 suppresses head and neck tumor growth. J Control Release. 2012; 159: 384-392. Ref.: https://goo.gl/yx5PYD
- Silva AC, Amaral MH, Lobo JM, Lopes CM. Lipid nanoparticles for the delivery of biopharmaceuticals. Curr Pharm Biotechnol. 2015; 16: 291-302. Ref.: https://goo.gl/btimzW
- Ravi S, Peh KK, Darwis Y, Murthy BK, Singh TR, et al. Development and Characterization of Polymeric Microspheres for Controlled Release Protein Loaded Drug Delivery System. Indian J Pharm Sci. 2008; 70: 303-309. Ref.: https://goo.gl/BuxNDy
- Champion JA, Mitragotri S. Role of target geometry in phagocytosis. Proc Natl Acad Sci USA. 2006; 103: 4930-4934. Ref.: https://goo.gl/uQGih6
- Radomsky ML, Whaley KJ, Cone RA, Saltzman WM. Macromolecules released from polymers: diffusion into unstirred fluids. Biomaterials. 1990; 11: 619-624. Ref.: https://goo.gl/C5jehN
- Sandor M, Enscore D, Weston P, Mathiowitz E. Effect of protein molecular weight on release from micron-sized PLGA microspheres. J Control Release. 2001; 76: 297-311. Ref.: https://goo.gl/MNgRDU
- Liggins RT, Burt HM. Paclitaxel loaded poly(L-lactic acid) microspheres: properties of microspheres made with low molecular weight polymers. Int J Pharm. 2001; 222: 19-33. Ref.: https://goo.gl/SqiiMc
- Malyala P,Singh M.Micro/nanoparticle adjuvants: preparation and formulation with antigens. Methods Mol Biol. 2010; 626: 91-101. Ref.: https://goo.gl/pw45cr
- Leleux J, Roy K. Micro and nanoparticle-based delivery systems for vaccine immunotherapy: an immunological and materials perspective. Adv Healthc Mater. 2013; 2: 72-94. Ref.: https://goo.gl/yqAd8Z
- Zhao L, Arjun Seth, Nani Wibowo, Chun-Xia Zhao, Neena Mitter, et al. Nanoparticle vaccines. Vaccine. 2014; 32: 327-337. Ref.: https://goo.gl/8dh7mX
- Kirby DJ, Rosenkrands I, Agger EM, Andersen P, Coombes AG, et al. PLGA microspheres for the delivery of a novel subunit TB vaccine. J Drug Target. 2008; 16: 282-293. Ref.: https://goo.gl/K1xKsL
- Lin CY, Lin SJ, Yang YC, Wang DY, Cheng HF, et al. Biodegradable polymeric microsphere-based vaccines and their applications in infectious diseases. Hum Vaccin Immunother. 2015; 11: 650-656. Ref.: https://goo.gl/Hw86LA
- Chaudhari KR, Ukawala M, Manjappa AS, Kumar A, Mundada PK, et al. Opsonization, biodistribution, cellular uptake and apoptosis study of PEGylated PBCA nanoparticle as potential drug delivery carrier. Pharm Res. 2012; 29: 53-68. Ref.: https://goo.gl/sxQ5ya
- Nance EA, Woodworth GF, Sailor KA, Shih TY, Xu Q, et al. A dense poly(ethylene glycol) coating improves penetration of large polymeric nanoparticles within brain tissue. Sci Transl Med. 2012; 4: 149-119. Ref.: https://goo.gl/7B26gE
- Sykes EA, Chen J, Zheng G, Chan WC. Investigating the impact of nanoparticle size on active and passive tumor targeting efficiency. ACS Nano. 2014; 8: 5696-5706. Ref.: https://goo.gl/4hEZk5
- Baker R. Controlled release of biologically active agents. Wiley Interscience Publications. 1987.
- Baxter J, Mitragotri S. Needle-free liquid jet injections: mechanisms and applications. Expert Rev Med Devices. 2006; 3: 565-574. Ref.: https://goo.gl/gmktFa
- Engwerda EE, Abbink EJ, Tack CJ, de Galan BE. Improved pharmacokinetic and pharmacodynamic profile of rapid-acting insulin using needle-free jet injection technology. Diabetes Care. 2011; 34: 1804-1808. Ref.: https://goo.gl/ic1qG2
- Jackson LA, Austin G, Chen RT, Stout R, DeStefano F, et al. Safety and immunogenicity of varying dosages of trivalent inactivated influenza vaccine administered by needle-free jet injectors. Vaccine. 2001; 19: 4703-4709. Ref.: https://goo.gl/D9XVqd
- Daniels CS. Needle-Free Injection: Pros and Cons. High Plains Dairy Conference. 2010; 25-36. Ref.: https://goo.gl/x5WHqs
- Stachowiak JC, Li TH, Arora A, Mitragotri S, Fletcher DA. Dynamic control of needle-free jet injection. J Control Release. 2009; 135: 104-112. Ref.: https://goo.gl/vb3Tkh
- Taberner A, Hogan NC, Hunter IW. Needle-free jet injection using real-time controlled linear Lorentz-force actuators. Med Eng Phys. 2012; 34: 1228-1235. Ref.: https://goo.gl/VCFrd1
- Kontermann R. Therapeutic Proteins: Strategies to Modulate Their Plasma HalfLives. Wiley-VCH: Verlag GmbH. 2012.
- Mero A, Pasqualin M, Campisi M, Renier D, Pasut G. Conjugation of hyaluronan to proteins. Carbohydr Polym. 2013; 92: 2163-2170. Ref.: https://goo.gl/KiiPdd
- Zhao H, Yang K, Martinez A, Basu A, Chintala R, et al. Linear and branched bicin linkers for releasable PEGylation of macromolecules: controlled release in vivo and in vitro from mono- and multi-PEGylated proteins. Bioconjug Chem. 2006; 17: 341-351. Ref.: https://goo.gl/vsuY1J
- Riggs-Sauthier J, Riley T. The Benefits and Challenges of PEGylating Small Molecules. Pharmaceutical Technology. 2008.
- Peters T. All about albumin. Academic Press. 1995.
- Kermode M. Unsafe injections in low-income country health settings: need for injection safety promotion to prevent the spread of blood-borne viruses. Health Promot Int. 2004; 19: 95-103. Ref.: https://goo.gl/n1HP3d
- Schaepelynck P, Darmon P, Molines L, Jannot-Lamotte MF, Treglia C, et al. Advances in pump technology: insulin patch pumps, combined pumps and glucose sensors, and implanted pumps. Diabetes Metab. 2011; 37: 85-93. Ref.: https://goo.gl/puzJ4o
- Ricotti L, Assaf T, Dario P, Menciassi A. Wearable and implantable pancreas substitutes. J Artif Organs. 2013; 16: 9-22. Ref.: https://goo.gl/iHMNLA
- Erasmo Lopez A, Atif Yardimci. Designing and Manufacturing Biopharma Delivery Devices. MDDI. 2015. Ref.: https://goo.gl/m6LNfm
- Lopez I, Rodríguez-Ortiz ME, Almadén Y, Guerrero F, de Oca AM, et al. Direct and indirect effects of parathyroid hormone on circulating levels of fibroblast growth factor 23 in vivo. Kidney Int. 2011; 80: 475-482. Ref.: https://goo.gl/Mg7Wvc
- Farra R, Sheppard NF Jr, McCabe L, Neer RM, Anderson JM, et al. First-in-human testing of a wirelessly controlled drug delivery microchip. Sci Transl Med. 2012; 4: 122ra21. Ref.: https://goo.gl/L7yEk2
- Zisser H, Palerm CC, Bevier WC, Doyle FJ 3rd, Jovanovic L. Clinical update on optimal prandial insulin dosing using a refined run-to-run control algorithm. J Diabetes Sci Technol. 2009; 3: 487-491. Ref.: https://goo.gl/JNi9x3
- Andrade F, Catarina M, Bruno S. Pulmonary Delivery of Biopharmaceuticals. Mucosal Delivery of Biopharmaceuticals. Springer. 2014. Ref.: https://goo.gl/d9Abw6
- Roth Y, Chapnik JS, Cole P. Feasibility of aerosol vaccination in humans. Ann Otol Rhinol Laryngol. 2003; 112: 264-270. Ref.: https://goo.gl/oyUSWY
- Lu D, Hickey AJ. Pulmonary vaccine delivery. Expert Rev Vaccines. 2007; 6: 213-226. Ref.: https://goo.gl/HkPkh5
- Arora P, Sharma S, Garg S. Permeability issues in nasal drug delivery. Drug Discov Today. 2002; 7: 967-975. Ref.: https://goo.gl/6AFGGE
- Illum L. Nanoparticulate systems for nasal delivery of drugs: a real improvement over simple systems. J Pharm Sci. 2007; 96: 473-483. Ref.: https://goo.gl/Q3fkvt
- Dae-Duk K. In vitro Cellular Models for Nasal Drug Absorption Studies. Drug absorption studies. 2007; 216-234. Ref.: https://goo.gl/tRDXrJ
- Kao HD, Traboulsi A, Itoh S, Dittert L, Hussain A. Enhancement of the systemic and CNS specific delivery of L-dopa by the nasal administration of its water soluble prodrugs. Pharm Res. 2000; 17: 978-984. Ref.: https://goo.gl/pfQgz2
- Yuba E, Kono K. Nasal Delivery of Biopharmaceuticals. Mucosal Delivery of Biopharmaceuticals. Springer. 2014; 197-220. Ref.: https://goo.gl/97LY9x
- Dahl AR, Lewis JL. Respiratory tract uptake of inhalants and metabolism of xenobiotics. Annu Rev Pharmacol Toxicol. 1993; 33: 383-407. Ref.: https://goo.gl/EtYPbp
- Mitra AK, Krishnamoorthy R. Prodrugs for nasal drug delivery. Adv Drug Deliv Rev. 1998; 29: 135-146. Ref.: https://goo.gl/3MW4mR
- Nema T, Jain A, Hurkat P, Shilpi S, Gulbake A, et al. Insulin delivery through nasal route using thiolated microspheres. Drug Deliv. 2013; 20: 210-215. Ref.: https://goo.gl/R66UHj
- Coucke D, Schotsaert M, Libert C, Pringels E, Vervaet C, et al. Spray-dried powders of starch and crosslinked poly(acrylic acid) as carriers for nasal delivery of inactivated influenza vaccine. Vaccine. 2009; 27: 1279-1286. Ref.: https://goo.gl/wJszFr
- Jabbal-Gill I. Nasal vaccine innovation. J Drug Target. 2010; 18: 771-786. Ref.: https://goo.gl/KEMrXm
- de Boer AG, Moolenaar F, de Leede LG, Breimer DD. Rectal drug administration: clinical pharmacokinetic considerations. Clin Pharmacokinet. 1982; 7: 285-311. Ref.: https://goo.gl/ceXzJv
- Kozlowski PA, Williams SB, Lynch RM, Flanigan TP, Patterson RR, et al. Differential induction of mucosal and systemic antibody responses in women after nasal, rectal, or vaginal immunization: influence of the menstrual cycle. J Immunol. 2002; 169: 566-574. Ref.: https://goo.gl/fFYfyq
- Pechine S, Denève C, Le Monnier A, Hoys S, Janoir C, et al. Immunization of hamsters against Clostridium difficile infection using the Cwp84 protease as an antigen. FEMS Immunol Med Microbiol. 2011; 63: 73-81. Ref.: https://goo.gl/g19EuJ
- Czerkinsky C, Holmgren J. Mucosal delivery routes for optimal immunization: targeting immunity to the right tissues. Curr Top Microbiol Immunol. 2012; 354: 1-18. Ref.: https://goo.gl/stkkJ2
- Rothbard JB, Garlington S, Lin Q, Kirschberg T, Kreider E, et al. Conjugation of arginine oligomers to cyclosporin A facilitates topical delivery and inhibition of inflammation. Nat Med. 2000; 6: 1253-1257. Ref.: https://goo.gl/osPdjf
- Medi BM, Singh J. Electronically facilitated transdermal delivery of human parathyroid hormone (1-34). Int J Pharm. 2003; 263: 25-33. Ref.: https://goo.gl/2Pqk4d
- Rastogi R, Anand S, Dinda AK, Koul V. Investigation on the synergistic effect of a combination of chemical enhancers and modulated iontophoresis for transdermal delivery of insulin. Drug Dev Ind Pharm. 2010; 36: 993-1004. Ref.: https://goo.gl/UhSyyJ
- Alba N, Naik A, Guy RH, Kalia YN. Effect of charge and molecular weight on transdermal peptide delivery by iontophoresis. Pharm Res. 2005; 22: 2069-2078. Ref.: https://goo.gl/7Nhmbd
- Abrego G, Alvarado H, Souto EB, Guevara B, Bellowa LH, et al. Biopharmaceutical profile of hydrogels containing pranoprofen-loaded PLGA nanoparticles for skin administration: In vitro, ex vivo and in vivo characterization. Int J Pharm. 2016; 501: 350-361. Ref.: https://goo.gl/JXBsZv
- Morishita M, Peppas NA. Is the oral route possible for peptide and protein drug delivery? Drug Discov Today. 2006; 11: 905-910. Ref.: https://goo.gl/Uq5CsC
- Whitehead K, Shen Z, Mitragotri S. Oral delivery of macromolecules using intestinal patches: applications for insulin delivery. J Control Release. 2004; 98: 37-45. Ref.: https://goo.gl/SgaEoQ
- Gupta V, Hwang BH, Lee J, Anselmo AC, Doshi N, et al. Mucoadhesive intestinal devices for oral delivery of salmon calcitonin. J Control Release. 2013; 172: 753-762. Ref.: https://goo.gl/YGt9St
- Collnot EM, Ali H, Lehr CM. Nano- and microparticulate drug carriers for targeting of the inflamed intestinal mucosa. J Control Release. 2012; 161: 235-246. Ref.: https://goo.gl/heTvs9
- Pridgen EM, Alexis F, Kuo TT, Levy-Nissenbaum E, Karnik R, et al. Transepithelial transport of Fc-targeted nanoparticles by the neonatal Fc receptor for oral delivery. Sci Transl Med. 2013; 5: 213ra167. Ref.: https://goo.gl/tQe99r
- McGinity JW, Stavchansky SA, Martin A. Bioavailability in Tablet Technology. Marcel Dekker: New York. 1981.
- Gaudana R, Ananthula HK, Parenky A, Mitra AK. Ocular drug delivery. AAPS J. 2010; 12: 348-360. Ref.: https://goo.gl/R7yzU1
- Ghate D, Edelhauser HF. Ocular drug delivery. Expert Opin Drug Deliv. 2006; 3: 275-287. Ref.: https://goo.gl/wZqzSE
- DEWS. The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007; 5: 93-107. Ref.: https://goo.gl/BKwcfc
- Abrego G, Alvarado H, Souto EB, Guevara B, Bellowa LH, et al. Biopharmaceutical profile of pranoprofen-loaded PLGA nanoparticles containing hydrogels for ocular administration. Eur J Pharm Biopharm. 2015; 95: 261-270. Ref.: https://goo.gl/nUJyDB
- Peng L, Cheng X, Zhuo R, Lan J, Wang Y, et al. Novel gene-activated matrix with embedded chitosan/plasmid DNA nanoparticles encoding PDGF for periodontal tissue engineering. J Biomed Mater Res A. 2009; 90: 564-576. Ref.: https://goo.gl/dDBoU4
- Elangovan S, Jain S, Tsai PC, Margolis HC, Amiji M. Nano-sized calcium phosphate particles for periodontal gene therapy. J Periodontol. 2013; 84: 117-125. Ref.: https://goo.gl/M7LX6N
- Torchilin V. Intracellular delivery of protein and peptide therapeutics. Drug Discov Today Technol. 2008; 5: 95-103. Ref.: https://goo.gl/wFPAkj
- Fu J, Yu C, Li L, Yao SQ. Intracellular Delivery of Functional Proteins and Native Drugs by Cell-Penetrating Poly(disulfide)s. J Am Chem Soc. 2015; 137: 12153-12160. Ref.: https://goo.gl/33R2zX
- Zolot RS, Satarupa B, Ryan Million P. Antibody-drug conjugates. Nature Reviews Drug Discovery. 2013; 12: 259-260. Ref.: https://goo.gl/JfS5Cd
- He C, Yin L, Tang C, Yin C. Size-dependent absorption mechanism of polymeric nanoparticles for oral delivery of protein drugs. Biomaterials. 2012; 33: 8569-8578. Ref.: https://goo.gl/p7zViN
- Wu SY, Lopez-Berestein G, Calin GA, Sood AK. RNAi therapies: drugging the undruggable. Sci Transl Med. 2014; 6: 240-247. Ref.: https://goo.gl/qqMdDN