Research Article
Nutritional Value of Three Different Oyster Mushrooms Grown on Cattail Weed Substrate

Ram Naraian1* and Bharti Dixit2
1Department of Biotechnology, Mushroom Training & Research Centre (MTRC), Faculty of Science, Veer Bahadur Singh Purvanchal University, Jaunpur-222003, Uttar Pradesh, India
2Institute of Paramedical Sciences, Chhatrapati Shahu Ji Maharaj University, Kanpur-208024, Uttar Pradesh, India
*Address for Correspondence: Dr. Ram Naraian, Department of Biotechnology, Mushroom Training & Research Centre (MTRC) Faculty of Science, Veer Bahadur Singh Purvanchal University, Jaunpur-222003, Uttar Pradesh, India, Tel: +91-9453095777; Email: [email protected]
Dates: Submitted: 16 August 2017; Approved: 30 August 2017; Published: 31 August 2017
How to cite this article: Naraian R, Dixit B. Nutritional Value of Three Different Oyster Mushrooms Grown on Cattail Weed Substrate. Arch Biotechnol Biomed. 2017; 1: 061-066. DOI: 10.29328/journal.abb.1001006
Copyright License: © 2017 Naraian R, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Pleurotus spp.; Biological efficiency; Protein; Fat; Crude fiber
Abstract
Three distinct oyster mushroom strains including Pleurotus florida (PF), Pleurotus eous (PE) and Pleurotus sajor-caju (PS) were successfully cultivated on cattail weed substrate. A comparative analysis of different parameters viz., biological efficiency (BE) and protein, carbohydrate, crude fiber and fat content in fruitbodies were evaluated. According to biological efficiencies obtained PF (90%) was superior strain, while order can be represented as 90%> 89%> 82% respectively in PF>PS>PE. The highest protein (4.8 g), lipid (0. 61 g) and dietary fiber (31.6g) values were obtained in the fruitings of Pleurotus sajor-caju. However, the utmost level of carbohydrate (41g) was recorded in Pleurotus eous. On the basis of the observation of the present study we recommend use of cattail weed substrate for cultivation of oyster mushrooms for better nutrients.
Introduction
Mushrooms having magnificent medicinal, delicacy as well as nutritive values are interestingly used as human food from the time everlasting. The fruiting bodies or mycelia are used as food and food-flavoring material for centuries due to their vast diversity of bioactive components [1]. Mushrooms normally ranges between the values of 20 and 40% protein, which ranks them better than many common legume sources like soybeans, peanuts, and protein-yielding vegetables [2,3]. Moreover, mushroom proteins specially contain essential amino acids needed in human diet and are especially rich in lysine and leucine, which lacks in most cereal foods [2]. Besides, mushrooms are rich in multiple minerals, and vitamins, and they contain an abundance of essential amino acids.
Pleurotus is a versatile genus belonging to white-rot basidiomycete fungi and well known for their complexity of the enzymatic system and prominent lignocellulolytic property, member of this genus can colonize a wide range of natural lignocellulosic wastes [1,4]. Apart from flavour and taste, the fruiting bodies of mushrooms are considered as sources of organic nutrients such as digestible proteins, carbohydrates, fibre and certain vitamins, as well as minerals and antioxidants [5]. Therefore, Pleurotus is one of the second most cultivated mushrooms and cultivated all over world.
Cultivation of several species of Pleurotus is cheapest and easiest to grow compared to all the cultivated edible mushrooms. Cultivation does not need difficult substrate preparation technique and former can be grown on ordinary lignocellulosic agro industrial residues containing lignin, cellulose and hemicelluloses. Oyster mushrooms are efficient lignin degraders which can grow on wide variety of agricultural wastes with broad adaptability to varied agro-climatic conditions [6]. For both spawn running and fruit body development lignin and cellulose materials such as corn cobs, all cereal straws, paper, wood shavings, sawdust, nutshells and vegetable wastes as well as food industry wastes are sufficient [7,8]. The present work was designed to compare biological efficiencies and nutritional status of three different species of oyster mushrooms cultivated on cattail weed substrate.
Materials and Methods
Mushroom strains and their maintenance
Three different oyster mushrooms including Pleurotus florida (PF), Pleurotus sajor-caju (PS) and Pleurotus eous (PE) were employed in present study. The cultures were obtained from Mushroom Training and Research Centre (MTRC), Veer Bahadur Singh Purvanchal University, Jaunpur, UP, India. It was maintained on Potato Dextrose Agar (peeled, sliced and boiled potato, 200 g; dextrose, 20 g; agar, 20 g l-1). The organism was maintained by sub-culturing fortnightly on the above said media at 22 ±1oC in culture tubes.
Substrate and its preparation
The cattail weed substrate was collected locally, and sun dried. The dried substrate was soaked into water in plastic drums. Two percent (v/v) formalin and 0.5 % (w/v) bavistin were used to kill contaminants in the water drum containing cattail weed substrate. After adding together of formalin and bavistin, the mouth of drum was wrapped and tightened using polythene sheet to prevent release of fumes and kept for overnight. After that the soaked cattail weed substrate was taken out of water and spread over formalin washed cemented floor to drain off excess water.
Spawn preparation
Spawn was prepared using wheat grains, which were initially soaked in tap water and slightly boiled up to kill seed. Later on the grains were thoroughly mixed with 2% (w/w) gypsum and 4% (w/w) calcium carbonate, then filled into wide mouthed glass bottles to two third of their capacities and cotton plugged. The cotton plugged bottles were then autoclaved at 20 psi. for 60 min. The bottles were shaken to break clumps of the grains and cooled overnight. Furthermore, sterilized and cooled bottles were inoculated with fungal discs and incubated at 18±10C in a BOD incubator. During the incubation, bottles were shaken daily, to uniformly mix fungal mycelia and cover around the grains.
Cultivation of mushroom and harvesting
The cultivation of mushroom was performed in polythene bags of standard size (18”×22”) by spawning through layer wise and bags were stored in dark at 23-250C till complete mycelia run. After complete mycelial run, polythene bags were gently removed which was followed to irrigation by water sprinkling and continued regularly at least thrice a day to keep moistened the beds. The room atmosphere was maintained with a relative humidity between the ranges of 70-90%. The fruitbodies of appropriate size before they become over matured were harvested with the help of sharp knife separately for each set and weighed.
Determination of biological efficiency (BE)
The cumulative biological efficiency was calculated as % BE with respect to Kg fresh mushroom Kg-1 dry substrate used.
Determination of protein, carbohydrate, lipid and crude fiber in fruitbodies
Total protein content present in fruitbodies was analyzed by the standard method of Bradford [9]. The solution of Coomassie Brilliant Blue G-250 dye was employed and absorbance was measured at 595 nm. The protein concentration was finally determined using standard curve plotted for bovine serum albumin (BSA) and calculated for g/100 g of the fruitbody sample. Total carbohydrate content available in fruitbodies was determined using the phenol-sulfuric acid method of Dubois et al. [10]. Total 500 µl sample was mixed with 500 µl 5% phenol followed by the addition of 2 ml sulfuric acid to the mixture and incubated for 20 minutes at room temperature. The absorbance was measured at 470 nm using spectrophotometer, and the total sugar was calculated by glucose standard curve. However, total amount of lipid was analyzed by the standard method by Folch et al. [11]. To analyze total lipid; 5 gram of each mushroom sample was suspended in 50ml mixture of chloroform: methanol (2:1) and mixed thoroughly. The preparation was kept as such to stand for 3 days. The solution was filtered, centrifuged (1000g, 10 min), upper layer of methanol was removed using Pasteur pipette, and crude lipid was collected to follow gravimetric analysis. The content of crude fiber was determined according to standard procedures of the AOAC [12].
Result and Discussion
Biological efficiency of three oyster mushrooms
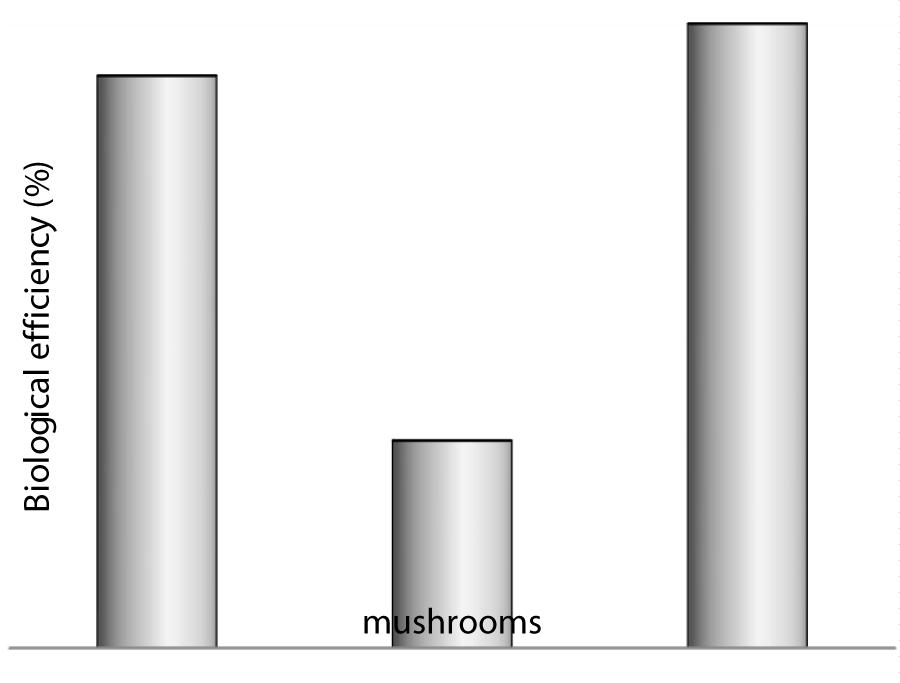
Biological efficiencies of three different oyster mushrooms grown on cattail weed substrate were evaluated. The highest 90% biological efficiency was achieved by Pleurotus florida which was followed by 89% in Pleurotus sajur-caju. However, lowest 82% biological efficiency was obtained in the set of Pleurotus eous (Figure 1). The order of biological efficiencies can be represented as 90%> 89%> 82% in PF>PS>PE respectively. Basak et al. [13] reported highest BE of 66.0% with the combination of rice straw–jute stick substrate. However, the combined harvest from two flushes of P. ostreatus grown on grass and coffee pulp produced a BE that varied between 59.8 and 93% [14]. In addition Zhang et al. [15] reported 128 and 97% BE by P. sajor-caju cultivated on rice straw and wheat straw, respectively. In a different study, the maximum biological efficiency (93.75%) of P. florida was achieved with CSC supplementation [16]. Moreover, similar observations were also reflected in several other studies [17,18].
Figure 1: Biological efficiency of three different oyster mushrooms cultivated on cattail weed substrate.
Crude protein content in fruiting bodies
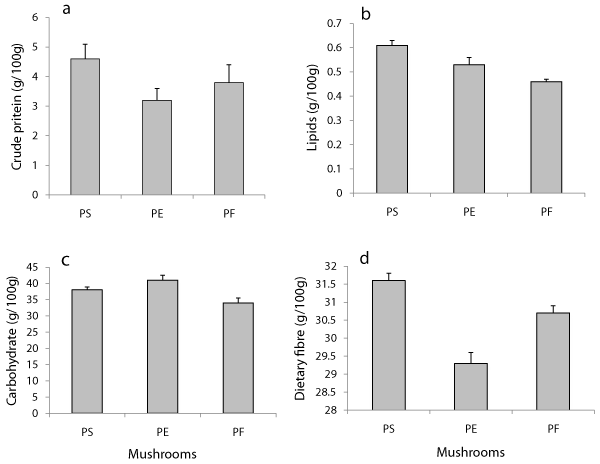
The nutritional value of mushrooms is generally rated in terms of their protein content in their fleshy edible fruitings. As compared the mushroom protein is ranked to have higher nutritional quality than that of plant proteins [19]. Generally, mushrooms being good source of protein they contain their protein contents ranging from 19 to 35% on dry weight basis [20]. In our study; crude protein in the fruiting bodies of three Pleurotus species were comparatively analyzed. It was found that Pleurotus sajor-caju contained highest 4.8 g protein, which was followed 3.8 g in Pleurotus florida. However, Pleurotus eous represented its lowest 3.2 g crude protein (Figure 2a). Based on the results obtained there was remarkable difference in the protein contents of three distinct species studied in present work. According to previous studies favoring our observations; protein contents of mushrooms are not only dependent on environmental factors and stage of fruiting body maturity, but also differs in distinct species of mushrooms [21,5]. Moreover, similar amounts of protein content were also reported by other workers [22,23] with P. florida and P. ostreatus. In a previous different study, we had observed resembling findings of protein which were in between 2.48-3.29g and 2.26-3.03g respectively in P. florida and P.sajor-caju cultivated on wheat straw and corn cob substrates in combination [24].
Lipid content in fruiting bodies
Oyster mushrooms are generally low in fat content, but contain some essential fatty acids for humans. However, they are not considered as a significant source of essential fatty acids for fulfilling the requirements of human body [25]. Though, are low in total fat content but have a high level of polyunsaturated fatty acid mainly linoleic acid. The lipid content in different species of Pleurotus species variably ranges from 0.2 to 8g per 100g dried fruit bodies, which have been reported from different studies [26]. The high content of linoleic acids is one of the reasons why mushrooms are considered a health food [3,27]. In present study comparative analyses of lipid level in fruiting bodies of three distinct Pleurotus species were performed. The analyses showed utmost level of lipid (0. 61 g) in the fruitings of Pleurotus sajor-caju mushroom, in contrast fruiting of Pleurotus eous represented second most highest lipid content (0.53g). The lowest level of lipid content was recorded in the fruitings of Pleurotus florida mushroom; which was as 0.46g (Figure 2b). In a similar observation Alam et al. [28] found similar results while evaluating P. florida and P. sajor‑caju.
Carbohydrate content in fruiting bodies
Carbohydrates are the important constituents found in mushrooms which provide energy and digestible carbohydrates in foods [29]. Mushrooms contain significant amounts of carbohydrates and fibres [22,2]. In this study total carbohydrate content in the fruitbodies of three different mushrooms were recorded. The highest crbaohydrate content (41g) was found in Pleurotus eryngii which was followed by 38g in Pleurotus sajor-caju. However lowest most carbohydrate recorded as 34g in the fruiting of Pleurotus florida . The order of carbohydrate was recorded as: 41>38>34g respectively in PE>PS>PE (Figure 2c). Simmilar to our findings Dundar et al., [30] also reported carbohydrate in different Pleurotus mushrooms. Watanabe et al. [31] found the carbohydrate value as 47.9 g in 100 g dry matter which is higher than our results.
Total dietary fiber in fruiting bodies
Crude fiber is a group of few indigestible carbohydrates and their availability makes mushrooms special in terms of neutraceutical and pharmaceutical uses. Mushrooms are a potential source of dietary fibers due to the presence of non-starch polysaccharides [25]. The fruting bodies of all three oyster mushrooms in present study contained appropriate amounts of dietary fibers. As observed; PS contained highest 31.6g of dietary fiber followed by 30.7g in PF and lowest in PE (29.3g). The order of dietary fiber can be represented as 31.6>30.7>29,3g respectively in PS> PF> PE (Figure 2d). However, 34.8% value of dietary fiber was reported in P. ostreatus cultivated on wheat straw substrate [32]. According to a study dietary fiber in P. ostreatus mushroom ranges from 4.1 g [33], which is very lesser to our findings in the present study.
Figure 2: Level of different nutritional components in the fruiting bodies of three different oyster mushrooms (a) crude protein (b) lipid (c) carbohydrate and (d) dietary fibre.
Acknowledgements
Authors wish to acknowledge the financial support of UGC, New Delhi major research Project (F.No.41-513/2012 (SR). The frequent encouragement of Prof. D.D.Dubey is highly acknowledged.
References
- Naraian R, Singh MP, Ram S. Supplementation of basal substrate to boost up substrate strength and oyster mushroom yield: an overview of substrates and supplements. Int J Curr Microbiol App Sci. 2016; 5: 543-553. Ref.: https://goo.gl/SSHFLg
- Chang ST, Buswell JA. Mushroom nutriceuticals. World J Microbiol Biotechnol. 1996; 12: 473-476. Ref.: https://goo.gl/EFVjRn
- Chang ST, Mshigeni KE. Mushroom and their human heal th:their growing significance as potent dietary supplements. The University of Namibia. Windhoek, 2001; 1-79.
- Naraian R, Singh MP. Improved yield of ligno-cellulolytic enzymes on oyster shell powder added typha weed substrate by Pleurotus florida. Cell Mol Biol. 2016; 62: 143-158.
- Wang XM, Zhang J, Wub LH, Zhao YL, Li T, et al. A mini-review of chemical composition and nutritional value of edible wild-grown mushroom from China. Food Chem. 2014; 151: 279-285. Ref.: https://goo.gl/NLAhL5
- Jandaik CL, Goyal SP. Farm and farming of oyster mushroom (Pleurotus sp). In: Mushroom Production Technology (Eds. Singh, R. P. and Chaube, H. S.). G. B. Pant Univ. Agril and Tech. Pantnagar India, 1995; 72-78.
- Yildiz A, Ertekin AS. An experimental study of mycelia development periods of some Pleurotus species. Mush Res. 1996; 5: 81-88.
- Baysal E, Peker H, Yalinkilic MK, Temiz A. Cultivation of oyster mushroom on waste paper with some added supplementary materials. Bioresour Technol. 2003; 89: 95-97. Ref.: https://goo.gl/WpDm5w
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Chem. 1976; 72: 248-254. Ref.: https://goo.gl/U9wLK9
- Dubois M, Gilles KA, Hamilton J K, Rebers PA, Smity F. Colorimetric method for determination of sugars and related substances. Analyt Chem. 1995; 28: 350-356.
- Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957; 226: 497-509. Ref.: https://goo.gl/tLW8Vn
- AOAC. Official methods of the Association of Official Analytical Chemists (16th ed). Arlington, VA, Association of Official Analytical Chemists. 1995.
- Basak MK, Chanda S, Bhaduri SK, Mondal SB, Nandi R. Recycling of jute waste for edible mushroom production. Ind Crop Prod. 1996; 5: 173-176. Ref.: https://goo.gl/FT14y1
- Hernandez D, Sanchez J, Yamasaki K. A simple procedure for preparing substrate for Pleurotus ostreatus cultivation. Bioresour Technol. 2003; 90: 145-150. Ref.: https://goo.gl/7RR2pT
- Zhang R, Li X, Fadel JG. Oyster mushroom cultivation with rice and wheat straw. Bioresour Technol. 2002; 82: 277-284. Ref.: https://goo.gl/XbMn2C
- Naraian R, Sahu RK, Kumar S, Garg SK, Singh CS, et al. Influence of different nitrogen rich supplements during cultivation of Pleurotus florida on corn cob substrate. Environmentalist. 2009; 29: 1-7. Ref.: https://goo.gl/5ZHZ1E
- Bano Z, Rajrathnam S. Studies on cultivation of Pleurotus sajor-caju. Mushroom News Lett Trop. 1983; 3: 12-15.
- Balakrishnan B, Nair MC. Production technology of oyster mushroom. In: Chadha KL, Sharma SR (eds) Advances inhorticulture, vol 13. Mushroom Malhotra Publishing House, New Delhi, 1995; 109-116.
- FAO (Food and Agriculture Organization). Protein quality evaluation Rome: Food and agricultural organization of the United Nations. 1991.
- Crisan EV, Sands A. Nutritional value. In S. T. Chang, & W. A. Hayes, The biology and cultivation of edible mushrooms.1978; 137-165.
- Colak A, Faiz Z, Sesli E. Nutritional composition of some wild edible mushrooms. Turkish J Biochem. 2009; 34: 25-31.
- Daba AS, Kabeil SS, Botros WA, El-Saadani MA. Production of mushroom (Pleurotus ostreatus) in Egypt as a source of nutritional and medicinal food. World J Agri Sci. 2008; 4: 630-634. Ref.: https://goo.gl/GqZKui
- Patil SS, Kadam RM, Shinde SL, Deshmukh SA. Effect of different substrate on productivity and proximate composition of P florida. Int J Plant Sci. 2008; 3: 151-153
- Naraian R, Srivastava J, Garg SK. Influence of dairy spent wash (DSW) on different cultivation phases and yield response of two Pleurotus mushrooms. Annals Microbiol. 2011; 61: 853-862. Ref.: https://goo.gl/2tJtwm
- Deepalakshmi K, Mirunalini S. Pleurotus ostreatus: an oyster mushroom with nutritional and medicinal properties. J Biochem Tech. 2014; 5: 718-726. Ref.: https://goo.gl/3eurEq
- Hossain MS, Alam N, Amin SMR, Basunia MA, Rahman A. Essential fatty acids content of Pleurotus ostreatus, Ganoderma lucidum and Agaricus bisporus. Bangladesh J Mush. 2007; 1: 1-7. Ref.: https://goo.gl/RWXWFU
- Sadler M. Nutritional properties of edible fungi. Br Nutr Found Nutr Bull. 2003; 28: 305-308. Ref.: https://goo.gl/AubXHt
- Alam N, Amin R, Khan A, Ara I, Shim MJ, et al. Nutritional analysis of cultivated mushrooms in Bangladesh-Pleurotus ostreatus, Pleurotus sajor-caju, Pleurotus florida and Calocybe indica. Mycobiol. 2008; 36: 228-232. Ref.: https://goo.gl/kj3cR4
- Vaz JA, Barros L, Martins A, Santos-Buelga C, Vasconcelos MH, et al. Chemical composition of wild edible mushrooms and antioxidant properties of their water soluble polysaccharidic and ethanolic fractions. Food Chem. 2011; 126: 610-616. Ref.: https://goo.gl/UvBFJS
- Dundar A, Acay H, Yildiz A. Effect of using different lignocellulosic wastes for cultivation of Pleurotus ostreatus (Jacq.) P. Kumm. On mushroom yield, chemical composition and nutritional value. African J Biotechnol. 2009; 8: 662-666. Ref.: https://goo.gl/wj16FQ
- Watanabe T, Tsuehihasi N, Takai Y, Tanaka K, Suziki A. Effects of ozone exposure during cultivation of oyster mushroom (Pleurotus ostreatus) on chemical components of the fruit bodies. J Jpn Soc Food Sci. Technol. 1994; 41: 705-708.
- Justo MB, Guzman GA, Mejia EG, Diaz CLG, Martinez G, et al. Calidad proteínica de tres cepas mexicanas de setas (Pleurotus ostreatus). Arch Latino de Nutri. 1999; 49: 81-85. Ref.: https://goo.gl/s5S4CR
- Manzi P, Aguzzi A, Pizzoferrator L. Nutritional values of mushrooms widely consumed in Itlay. Food chem. 2001; 73: 321-325. Ref.: https://goo.gl/ftj36z