More Information
Submitted: 15 October 2020 | Approved: 06 November 2020 | Published: 09 November 2020
How to cite this article: Chand A. Packaging challenge for COVID-19 drug. Arch Biotechnol Biomed. 2020; 4: 028-036.
DOI: 10.29328/journal.abb.1001019
Copyright License: © 2020 Chand A. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Packaging challenge for COVID-19 drug
Anupam Chand*
Post Graduate from IIP, Mumbai, India, Bioxytran. Inc, Boston, MA, USA
*Address for Correspondence: Anupam Chanda, Post Graduate from IIP, Mumbai, India, Bioxytran. Inc, Boston, MA, USA, Email: [email protected]
At present Corona virus is the most burning topic across the world. At present there is no drug available to cure 100%. So many companies are trying to make it possible as soon as they can. The basic characteristics of the product is “Non-Toxic made of sugars Carbohydrates”. A Galectin is a protein that recognizes carbohydrates and modulates intracellular and extracellular interactions primarily related to the immune system. In some cases Galectins act as a glue bringing molecules together. The major focus of the research is on extracellular interactions. Galectins have emerged as amplifiers or silencers of inflammatory responses, capable of orchestrating complex regulatory circuits in innate and adaptive immune cells, as well as in synovial fibroblasts. Galectins are responsible for T-cell energy and prevent LFA-1 lectins (depicted in blue) from coalescing and adhering to the target cell in order to destroy it with cytotoxins. The process of selecting materials and the type of packaging also offers an opportunity for the Packaging scientist to look for new biological delivery choices. Most injectable protein products were supplied in some sort of glass vial, prefilled syringe, and cartridge. That product having high Ph content there is a chance of “delamination “from inner surface of glass vial. With protein-based drugs, the biggest issue is the effect of packaging derivatives on the protein’s three-dimensional and surface structure. These are effects that relate to denaturation or aggregation of the protein due to oxidation or interactions from contaminants or impurities in the preparation. The potential for these effects needs to be carefully considered in choosing the container and the container closure system.
Purpose and special role
a) To develop commercially viable packaging along with product which common people can afford.
b) Selection of right kind of packaging material and innovative design which can be user friendly.
c) Packaging material has to be compatible with product.
d) Products and packages are optimally designed to meet their intended use. This applies especially to packaging materials, which should only be as durable as necessary to serve their intended purpose.
e) Control of packaging material is an art; require extensive effort and adequate experience. Health-care establishments are massive producers of waste.
Drugs
➣ Characterized sugar molecule.
➣ It needs to be dissolving at room temperature and after time to elevate the ph.
➣ The substance needs to be washed with ethanol and sterilized at Room temperature and aseptic filling in 50ml sterile vials.
➣ In the viral evolutionary path it appears that the Cove stole a host galectin gene and inserted it at the end of their spike gene. The S1-NTD Cove has a galectin fold that is likely shared amongst different Cove genus but they are programmed to recognize different sugar receptors. Theory suspects viral lectins originated from host galectins but evolved the galectin fold to evade the immune system.
➣ Bind to the virus, acting as an entry inhibitor.
➣ Binding to the virus soaks up the vision. The liver filters out the galectin inhibitor with vision still attached.
➣ Throttle the trafficking of macrophages to the site of infection.
➣ Remove plaque on T-cells resulting in improved response.
Perfect drug should have the following qualities:
➣ Prophylactic properties that allow a person to be exposed yet stop the viral expansion dead in its tracks.
➣ Drug must reduce the viral load of those who are infected.
➣ Stoppage of the trafficking of suppressor cells designed to silence the immune response.
Probable packaging Challenges:
a) Delamination of Glass and solutions
b) Protein absorption on glass and rubber surface and probable solution
c) Protein absorption in Needle
d) Extractable and Leachable from glass, tungsten needle(for PFS),rubber stopper and polymer
Delamination of Glass and Solution: Delamination of pharmaceutical glass is a serious issue, as it can cause glass particles to appear in vials, a problem that has forced a number of drug product recalls in recent years. In Type I pharmaceutical glass vials, delamination occurs generally at the bottom and shoulder, where extensive flaming during the conversion process can favor a strong evaporation of alkali and borate species and the formation of heavily enriched silica layers. The contact with parenteral preparations dissolved in an alkaline medium increases the rate of glass corrosion, while the differential hydration of these layers can cause the detachment of flakes. The effect of the pH and the composition of the extraction solutions on the propensity of different glass types to delaminate.
Different causes
What is Delamination and Reason
• Type I – Borosilicate Glass: An essential step in the manufacture of pharmaceutical vessels from borosilicate tubing is flame working of the tubing to shape and form the finished packaging. This flame working has been demonstrated to cause the release of boron and sodium from the glass surface, which then condenses onto the glass surface. There is increased prevalence of delamination at both the heel and shoulder of vials made in this process – further supporting a relationship to the flame working. The resulting effect is that the glass surface is left enriched with silica with loosely attached condensates that can either remain or fall into the product within the vessel.
• Type I– Soda-Lime-Silicate Glass: This product is stable in “ Alumino Silicate Glass also. We never face delamination problem in this glass vial. This is better than our conventional “Borosilicate Glass vial.
Different causes of delamination
a) Formulations with a high pH, and those that include phosphate and citrate buffers increase the risk of glass delamination,
b) Phosphate and citrate buffers can corrode glass, for example, the active molecule may require the attributes of those ingredients. “Any change to the formulation may affect the safety and efficacy of the product.
c) High alkali content in glass could accelerate erosion, so manufacturers should choose vials with low alkali content if possible.
d) High temperatures during the vial-forming process increase the risk of glass delamination. Appropriate control of temperatures during the glass-forming process is especially important when the containers will store corrosive product formulations. Drug manufacturers should ask vial vendors about their process control, and also test vials to ensure their suitability for the product in question.
e) Terminal sterilization also is a risk factor that, together with specific products, could cause delamination.
f) High product-storage temperatures and long exposure times can increase the rate and severity of glass delamination.
How to prevent delamination of glass
a) Treating the surface of the glass vials with materials, such as ammonium sulphate, can reduce the rate of glass erosion.
b) Consider alternative sterilization methods only in rare cases.
c) The correct specification for the glass to ensure its suitability for the pH of the product.The processing involved sterilization and autoclaving processes. Both handling and storage conditions for the filled product. Glass manufacturers ensuring that appropriate process controls are in place. Forming temperatures and correct annealing and due diligence in the quality testing and assurance of their glass.
d) Siliconization on inner surface of glass vial/PFS/cartridge.
e) Use of COC/COP vial.
f) Use Alumino Silicate Glass vial.
b) Protein absorption on glass and Rubber surface and probable solution: Adsorption of proteins to primary containers can result in protein loss, protein denaturation, or aggregation. The most effective method to directly detect and visualize adsorption of proteins to container surfaces by staining adsorbed proteins with gold nanoparticles, which bind proteins nonspecifically. The gold nanoparticle staining method was applied to study adsorption to siliconized glass prefilled syringes (PFSs) of a therapeutic protein in a liquid formulation. The protein was found to preferentially adsorb to glass surfaces over siliconized surfaces in PFSs. The presence of adsorbed proteins on glass surfaces was confirmed by in situ Raman spectroscopy. Gold nanoparticle staining patterns revealed that adsorption of proteins to hydrophobic cyclic olefin polymer plastic vials was minimized compared with hydrophilic type I glass vials.
Example of Delamination in Glass Vial (50 ml).
Delamination in Syringe.
Delamination in Syringe.
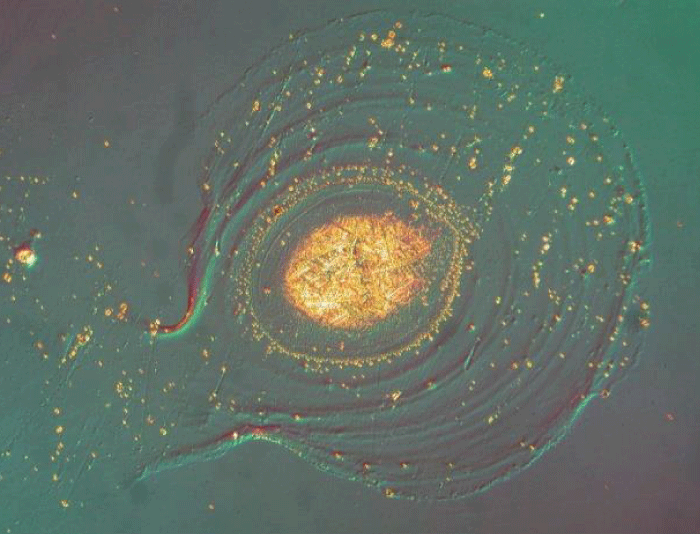
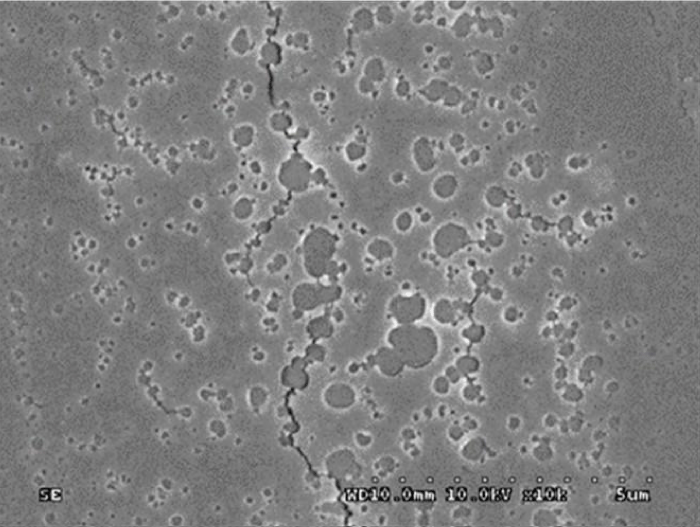
Glass delamination under the Carlton Microscope.
Optical coating degradation problem.
Delamination of Glass under Microscope.
Delamination of Glass under Microscope.
Protein loss and effects on quality of drugs
Suitability of a packaging system comprises the protection and compatibility with the dosage form, safety and proper performance function. Adsorption of the active drug substance and a related potency loss may constitute a compatibility problem if unacceptable quality changes take place. Therefore, stability testing is usually performed in the packaging system intended for final use. The majority of small volume injectable formulations are stored in vials, followed by syringes, ampoules and cartridges, whereas bottles and bags are used for larger volumes. Still, one of the most commonly used materials is glass, but also plastic vials and different coatings and surface modifications for special applications are available on the market.
| Glass Vial VS PFS (Glass). | |||
| Testing/manufacturing | Glass vial | PFS( Glass) | |
| Plasma/mass spectrometry, scanning electron microscopy, atomic force microscopy. | Observed delamination more | Observed delamination very less | |
| Glass processing history, including forming and annealing, sterilization and depyrogenation, and surface treatments. | Possibility of delamination is more | Possibility of delamination is very less. | |
| Formulation in contact with the container during its shelf life. | Is more | Less | |
| For tubing vials, heat is applied to cut and part the glass cane, then to tool the neck, and finally to form and polish the bottom. The most extreme heat is used for forming the vial bottom, the region just above the vial bottom to be more susceptible to delamination. | Delamination observed from neck and bottom | Delamination possibility is less | |
| Product “ Ph” value more than 6.5 | Recommending | Not recommending | |
| Few Practical Examples: Average Ph: 6.2 to 6.5 | ||
| Sl # | Do | Not to Do |
| 1 | Recommending “Tubular vial for “ 5ml “ capacity | As per stability result no need to go for “COC/COP “vial. Cost of COC/COP vial is very high compare to Glass vial. |
| 2 | Strongly recommending “moulded vial for 100 ml capacity” | Don’t use “Tubular vial for 100 ml capacity since “observed delamination during stability study. |
| Challenge in Production line and Probable Solutions. | ||
| Sl# | Problems | Probable solutions |
| 1 | Breakage of vial/PFS/cartridge | Proper handling is required while keeping packaging materials on round table and during washing in case of vial. |
| 2 | Improper filling of vial/PFS/cartridge | Right positioning of “Nozzle “is a must. Operator has to be efficient |
| 3 | Improper fitment of Rubber stopper | Rubber stopper “pick and place” makcanizam has to work properly. Air compressor need to check as well. |
| 4 | Improper sealing of vial | Seal inner diameter, sealing head, height and pressure need to check. |
| 5 | Leak test failure | Stoppering and sealing have to be perfect. Sealed vials need to check online or off line “leak tester”. |
| 6 | Leakage observed in PFS | During insertion of “plunger Rod” inside the “Plunger stopper” the height between plunger stoppering part and actual position of plunge need to increase in order to reduce pressure on plunger stopper. |
| 7 | Black particle and glass fibre in product | Need to monitor properly Onlineand off line inspection system. Periodic |
Protein absorption on glass
Borosilicate or Alumino Silicate Glass vial has a high chemical resistance, low leachability and a low thermal expansion coefficient is formable and tight toward gases and liquids. The thermal stability of this material allows a time and cost saving sterilization and depyrogenation by dry heat at high temperatures, which is a major advantage compared to plastics such as cyclic olefin copolymers (COC) or polypropylene. Although the glass surface is basically chemically resistant, reactions with water, acids and alkaline may lead to leaching and extraction of glass components. This loss of hydronium ions during leachingleads to rising pH values in the product solution and potential instabilities of biomolecules.
Alkaline attack of the glass surface which is also known as etching leads to the disintegration of the SiO network and finally to decomposition of the glass. Water leads to a leaching of ions and ion exchange between the solution and the glass. Finally, glass flaking may occur due to the delamination of glass which is embedded at the inner glass surface. These flakes consist of alkali borates which recondensed during the final glass preparation after migration to the inner surface and evaporation.
Siliconization and its advantage
1. Coatings with silicone provide advantages such as a good drainage of the solution from the vial wall and thus a better dosage, an easy movement of rubber plunger e. g. in feeding machines.
2. Reduction of the force for plunger movement in syringes .One of the most common silicone fluids applied for the coating of parenteral packaging components is Polydimethylsiloxane (PDMS).
Alternate to glass
Besides glass vials, plastic vials of cyclic olefins such as cyclic olefin copolymer (COC) andcyclic olefin polymer (COP) have been developed for the packaging of injectables .Compared to polypropylene and polyethylene, these materials provide advantages like hightransparency, the absence of extractable metal ions and low levels of organics which may be extracted. Although plastic containers exhibit advantages compared to glass vials e.g. aresistance to breakage and a reduced release of leachable (for COC and COP) their useis not very common. Some reasons are the higher costs of these vials and the challenges, arising from their transfer into an aseptic environment: In contrast to glass vials which areconveyed through a heating tunnel directly into an aseptic area, plastic vials are obtained presterilized(e.g. by irradiation) and have to be transferred aseptically. Furthermore, the lowweight of these vials compared to glass vials makes their transport on conveyers and their handling difficult, and the potential of product-container interactions over shelf-life periods of several years is still rising concerns. Another option to improve the properties of the packaging materials is the coating of a glass or plastic container. One common coating is the siliconization as presented in section.
Inner surface modification of glass (for reduction of protein absorption)
1. Coating of glass vials with thin layers of SiO2 by Plasma Impulse Chemical Vapour Deposition (PICVD) strongly reduces the interaction between the vial surface and the drug.
2. Poly- or oligoethylene glycol (PEG/OEG)-based coatings are one of the most popular and best investigated coatings. The excellent protein repellent effects of these coatings were ascribed to a steric repulsion or excluded volume mechanism or the formation of a structurally stable interfacial water layer.
3. Coatings with polyglycerols, zwitterionic self-assembled monolayers and polymers. Carbohydrate-derived polymers and dextran.Besides the ability to prevent the adsorption of proteins, a variety of other factors decide about the feasibility of a coating for industrial primary packaging materials for injectables.
Few options to reduce protein adsorption:
• The inclusion of high concentrations of an inert protein in the drug formulation to saturate the glass surface
• The addition of carbohydrates, surfactants or amino acids to reduce interaction between container surface and protein
• Silicone oil treatment of the glass vial surface to reduce the adsorptionM
• Use of multilayer COC or COP vial Glass is composed of a set of inorganic oxides that forms a threedimensional structure during the manufacturing process. Parenteral drug solutions with with a pH value > 7 attack the surface. Under such conditions the glass releases metal ions with potential adverse effect on the stability of sensitive biopharmaceuticals. In severe cases the attack on the glass surface can even cause flaking, which has led to an increased rate of recalls. pH shift or released metal ions would be sufficient to cause an adverse effect on the drug.It would denature a protein in a biopharmaceutical formulation. COP/COC based vials are an ideal solution to problems experienced in connection with parenteral drug solutions with high pH-value.
• Advantages of COP/COC vial
1. High gas barrier properties without the loss of any advantages such as low adsorption tendency.
2. No metal ion release
3. High transparency and high drain ability.
4. The drug contact surface area remains COP, which is known to be a very inert and clean material.
5. The advantage of the multilayer design is the increased oxygen barrier and improved integrity when the container suffers from external impact or incidental drop due to the extremely high puncture resistance of polyamide which strengthens the structure.
Challenge to design innovative PFS
Advantage of PFS
✓ Ease of use (and thus opportunity for self-administration or administration without further manipulations of the primary container)
✓ Reduction of medication errors.
✓ Product differentiation.
✓ Less overfill.
✓ Possibility to “combine” with an auto injector (in case of staked-in needle PFS).
Main challenges for a formulation scientist are related to:
• Syringe ability
• compatibility
Syringe ability
Time and force required for a manual injection (or time required for an injection using an auto injector) are important and may impact the usability of the product by the end-user and thus compliance. The force required for the injection of a solution at a given injection rate via a needle of predetermined gauge and length is referred to as ‘syringe ability’. The Hagen-Poiseuille equation can be utilized to estimate the travel (or glide) force (Equation 1).
Q = Volumetric flow rate
μ = Fluid viscosity
L = Needle length
R = Needle inner diameter
A = Cross sectional area of syringe plunger
F = Frictionless travel force
Calculated travel force as a function of needle inner diameter and dynamic viscosity for an injection rate of 0.1 mL/s using a syringe with an inner diameter of 4.6 mm and a needle of 20 mm length applying the Hagen-Poiseuille equation.
According to equation 1, the travel (or glide) force is dependent on a number of parameters. The only parameter a formulation scientist can influence is viscosity. All other parameters (needle inner diameter, needle length and cross sectional area of syringe plunger) are determined by the pre-fillable syringe itself. Formulations with a high viscosity can lead to high injection forces and long injection times since both parameters are proportional to viscosity. When developing a PFS for an auto injector, the limit for the injection force is determined by the auto injector technology. Thus, the technology applied needs to be taken into account for the overall design of the drug product-device combination product. The dimensional attributes of the pre-fi llable syringe need to be adjusted to the viscosity of the formulation and a close collaboration between pharmaceutical scientists and device engineers is mandatory when developing PFS and auto injector presentations.
Viscosity
The viscosity of a solution is dependent on the protein itself, the protein concentration, the temperature and the formulation, e.g. pH, type of excipients and excipient concentrations. The viscosities of different monoclonal antibodies at a given concentration can vary significantly even with minor differences in the amino acid sequence. In case the target product profile foresees a high concentration liquid formulation for subcutaneous administration (e.g. a monoclonal antibody in an inflammation indication); it is prudent to include viscosity as a criterion already during drug candidate selection/molecule assessment to identify early on potential issues for pharmaceutical development.
Besides the protein itself, protein concentration is another important factor for viscosity. Subcutaneous injections are generally limited to an injection volume of approx. < 1.5 mL. Depending on the absolute dose, high concentration liquid formulations may be required to avoid multiple injections. In fact, most monoclonal antibody-based products for monoclonal antibodies, the viscosity usually increases exponentially at a concentration of > 50 to 100 mg/Ml. During formulation development of a new biological entity, the final dose it usually not known. In this case, a protein concentration “as high as technically feasible” is usually aimed for when developing PFS presentations. The impact of the protein concentration on product stability needs to be assessed as well as the impact of viscosity on the drug substance and the drug product manufacturing process and on syringe ability. The formulation scientist needs to consider not only the viscosity at the nominal protein concentration but also the viscosity at the upper specific action limit (e.g. nominal protein concentration + 10%) and the protein concentration during DS manufacturing which can be significantly higher.
Compatibility
The number of compounds which may leach into the drug product solution is in sheer number- much higher for PFS compared to vial presentations as the number of components which are part of the container closure system is higher. Besides an assessment of (potentially) leaching components on patient safety, the potential to induce or accelerate protein degradation needs to be considered during formulation development.
Tungsten
Tungsten pins are routinely used by manufacturers of glass pre-fillable syringes in the syringe manufacturing process to keep the bore open while the cone is being shaped. The shaping process requires application of high temperatures. Although tungsten is quite resistant, metal particles may be deposited from the pins during the process. In addition, oxidation processes occur leading to formation of tungsten oxides that may subsequently deposit in the bore and funnel area of the syringe. In this respect it is important to keep in mind that deposits of tungsten species can occur in staked-in needle as well as luer type pre-fill able syringes (although staked-in needle pre-fillable syringes contain the lowest amount of extractable tungsten as most of the bore is covered by the needle.
When exposed to an aqueous solution with an acidic pH of about 6 or below, both tungsten metal and tungsten oxide can form a variety of soluble polyanionic species which can induce protein aggregation and formation of proteinaceous particles. Protein oxidation was reported to be induced by tungsten as well leading to protein aggregation. Other formulation parameters which have been reported to have an influence on tungsten induced protein degradation besides pH are protein concentration and ionic strength. Thus, pH, protein concentration and ionic strength of a formulation should be taken into account in the risk assessment for tungsten compatibility of a formulation.
Stability testing in the actual PFS can to a certain degree assess also compatibility with tungsten, however, with the caveat of variability of these deposits. Other approaches to assess tungsten compatibility are e.g. spiking with soluble tungsten species (e.g. Na2WO4), spiking with an extract from used tungsten pins or spiking with an extract from the glass barrel.
Silicone oil
Silicone oil or silicone oil emulsion is used to lubricate the inner wall of the glass barrels of pre-fillable syringes to ensure functionality of the PFS and/or auto injector over its shelf life.
Silicone oil has been inducing protein aggregation of some model proteins in non-optimized formulations under quiescent storage at 45°C and to increase protein aggregation upon agitation stress. However, the spiked silicone oil concentrations were much higher than typically observed in PFS. In any case, agitation stress studies of candidate formulations in PFS should be part of formulation screens in addition to quiescent stability studies.
A minimum silicone oil quantity is necessary to ensure functionality over shelf life of the product. Therefore reducing the silicone quantity per syringe to counteract silicone oil incompatibility may not be feasible or even a concern. Silicone-free plastic syringes may also not provide a suitable option as these systems are still not well established yet and are associated with other challenges (e.g. oxygen permeability, potential leachable and surface sensitivity).
Another challenge associated with silicone oil is related to the formation of silicone oil droplets in the sub-visible particle size range. Once the silicone oil detaches from the inner wall of the glass barrel, silicone oil droplets are formed and contribute to the sub-visible particle population. Silicone oil droplets can be found in PFS independent of the siliconization process.
Adhesives
Adhesives are utilized to attach the needle in staked-in needle pre-fillable syringes. The adhesive is usually cured by UV light. An incomplete UV curing process led to an unexpected impurity found in a new staked-in needle PFS presentation for a biological product which has not been previously observed in a luer cone PFS presentation. Process improvements by the syringe vendor could dramatically decrease the impurity levels in the syringe.
Needle shield rubber components
The root cause of clogged needles as protein aggregation which led to a gel-like clog. Induced protein aggregation was related to leachable from certain types of needle shields while others did not have any effect. Zinc plays a role but does not seem to be the only reason. Another option, decreased polysorbate concentration which could indirectly lead to instabilities.
Few important points:
• Reliably predict the viscosity based on primary structure and homology modelling using in silicon methods
• Increase the choice of viscosity reducing excipients
• Extend auto injector operating range with regards to viscosity
• Extend DS and DP manufacturing process operating ranges with regards to viscosity
• Explore the maximum tolerable subcutaneous injection volume
• Better predict human in vivo injection times based on in vitro models
• Better understand the impact of protein and formulation parameters on tungsten and silicone oil compatibility
• Improve capabilities of analytical methodologies for the characterization of sub-visible and submicron particles in terms of size limitations and in terms of particle type discrimination.
Challenge to design suitable plunger stopper
Impact of Residual Impurities and Contaminants on Protein Stability
A variety of expression systems can now be selected for production of proteins of interest with desired post-translational modifications. The expressed protein can be isolated and purified to a high degree traditionally through a combination of chromatographic steps. The purified protein is then put into a stable buffered matrix by ultra filtration/infiltration (UF/DF), and filled and possibly lyophilized in final drug product containers for commercialization. The multistep manufacturing processes for the protein drug substance and final drug product generate a variety of process-related impurities and provide many opportunities for potential product contamination. Product contamination can be classified generally into two categories—microbiological and no microbiological.
Fatty acids/lipids
Fatty acids and lipids can be present in the final drug substance. They are introduced as cell components during expression, as processing aids during purificationor during viral inactivation.Fatty acids and lipids could potentially influence the protein stability, as they can bind tightly to proteins, presumably through hydrophobic interactions. Design of a process to remove the residual fatty acids/lipids can be based on the purification of membrane proteins and/or pH adjustment method.
Metals/nonmetallic leachables
Most bioprocessing equipment is made of stainless steel in a variety of qualities, which may corrode to a different degree in solutions of different compositions, especially in chlorinated solutions. Three metal ions—iron; chromium, and nickel— were identified to be the major leachable under various formulation buffer and pH conditions. Both protein and ethylenediaminetetraacetic acid (EDTA) in a product formation increase the dissolution of stainless steel, and the metal leaching process. NaCl was also able to facilitate corrosion of certain stainless steel components in a low pH solution.
Organic solvents
Organic solvents have also been used as processing aid in certain cases.Solvent detergents are often used for virus inactivation and reduction. Trifluoroacetic acid is often used as a solvent in reversed-phase HPLC for purification of proteins or peptides. Therefore, residual organic solvents can be found in the protein drug substance. The effect of organic solvent on protein stability has been widely studiedThe effect can vary dramatically, depending on the concentration of the organic solvent and protein. A gradual effect is generally seen with increasing proportions of an organic solvent in proteins such as alpha-chymotrypsin with several organic solvents and human growth hormone with ethanol solutions. Simulation of the effect of different organic solvents (form amide, acetone, and isopropanol) at no denaturing concentration on the structure of haloalkane dehalogenases reveals that the behavior of solvent in the vicinity of hydrophobic patches on the protein surface is similar to the air/water interface. Therefore, it is conceivable that such organic solvents would facilitate formation of protein.
Silicone Oil: Silicone oil is commonly used to coat rubber stoppers to facilitate manufacturing operations or syringe barrels/plungers to facilitate both manufacturing and administration processes clear trend today is to market biological products in pre- filled syringes for better safety and ease of administration. An obvious concern is the potential long-term effect of silicone oil on protein stability during product storage.
Proteins can be easily adsorbed onto the silicone oil droplet surface, leading to a loss of soluble proteins. lysozyme, abatacept, and trastuzumab.
➣ The amount of protein absorbable on the surface of silicone oil may need further investigation, although at least a monolayer of protein molecules was demonstrated in some studies.
➣ The adsorption of protein at the silicone oil/water interface can be irreversible and protein concentration dependent.
➣ Siliconeinduced surface adsorption of proteins can facilitate protein aggregation and particle formation.
➣ Silicone-induced protein aggregation was shown to be pH-dependent, as pH regulates the surface charge and hydrophobicity of a protein.
➣ It is possible that the polysorbate in the formulation has certain protective effect. Several approaches can be taken for mitigation of the impact of silicone oil.
Challenge to select right kind of needle
Tungsten residues also need to be minimized. Tungsten pins are used during syringe manufacture to keep the bore open while the cone is shaped. As a result, metal and metal oxide particles may be deposited on the glass during the process. This residue can also lead to protein aggregation and formation of proteinaceous particles. Some suppliers have tried replacing tungsten pins with other materials, such as transition metals.
Extractable and leachables
Assessment of product leachable/extractable from a container/closure system is one of the key tasks during the drug development process and through product approval. Leachables from product container/closure systems can cause serious safety concerns.
➣ For example, leachable from the stoppers of PS 80 considered to have contributed to the increased incidence of pure red cell aplasia.
➣ Metal ions can leach from different glass or plastic packaging materials. The type and level of metal leachable are dependent on the type of containers, incubation temperature, and formulation composition
➣ Tungsten plays a key role in protein aggregation. This metal is used during syringe manufacturing and can leach out in significant amounts in the funnel region of a prefilled syringe and facilitate formation of protein particles. Tungsten at ppm levels was adequate to facilitate formation of subvisibleparticulates or precipitates in monoclonal antibody formulations
➣ Borosilicate vials could also interact directly with a protein product or form other complexes with formulation excipients to indirectly influence the stability of proteins.
➣ Glass surfaces can leach alkali components causing pH changes over time, especially under basic conditions.
Present market demand is syringe barrel and/or plunger coated with silicone. Non-uniform silicone coatings can affect protein stability. The proteins can absorb into the walls of the container. While silicone can reduce absorption, excess silicone can form suspended oil-like droplets. Proteins can form around those droplets and change their natural state. New lubricant coatings, such as inert fluoropolymers, are being introduced to replace silicone. As a very inert material, fluoropolymer provides smoothness for the syringe plunger without the irregularities or the issues that have come with silicone. Other new coating materials are being introduced with new types of packaging related to self-injectors, especially injector pens, patches, and transdermal and wearable devices for self-infusion. Extractable and leachable are most important for inhalers and catheters.
For an extractables from a device component the AET (μg/g) can be determined using Equation 1: Equation 1
AET = SCT.Dt
Dd m
Dd- Doses per day
Dt- Total Labelled doses
m - mass of component
The AET (μg/device) for a drug delivery device (e.g. an MDI) can be determined from Equation 2:
AET = SCT.Dt
Dd
Dd- Doses per day
Dt- Total Labelled doses
- www.pda.org
- www.glass-ts.com
- www.sciencedirect.com
- Highlights from June 2011 Glass Container Delamination Scientific Symposium. 2013. http://www.rx-360.org/LinkClick.aspx?
- Swift, Rob Amgen presentations, PDA April 2011 San Antonio; PDA/FDA Glass Quality Conference May 2011.
- http://www.fda.gov/Drugs/DrugSafety/ucm248490.htm
- Ennis RD, Pritchard R, Nakamura C, Coulon M, Yang T, et al. Glass vials for small volume parenterals: influence of drug and manufacturing processes on glass delamination. Pharm Dev Tech. 2001; 6: 393-405. https://pubmed.ncbi.nlm.nih.gov/11485181/
- Desmond H. USP Presentation, PDA/FDA Glass Quality Conference May 2013.
- https://www.sciencedaily.com/releases/2020/09/200915152448.htm
- https://youtu.be/FD0fhZB3bUE
- https://investor.regeneron.com/static-files/a596a85e-e72d-4529-8eb5-d52d87a99070
- https://www.businessinsider.com/regeneron-ceo-says-trump-antibody-drug-experience-weakest-evidence-success-2020-10
- https://www.cbsnews.com/news/transcript-regeneron-ceo-leonard-schleifer-on-face-the-nation-october-11-2020/