More Information
Submitted: April 20, 2021 | Approved: May 06, 2021 | Published: May 07, 2021
How to cite this article: Mantareva V, Syuleyman M, Slavova-Kazakova A, Angelov I, Durmus M. Mestranol moieties clicked to Zn(II) phthalocyanine for controllable photosensitized oxidation of cholesterol. Arch Biotechnol Biomed. 2021; 5: 041-048.
DOI: 10.29328/journal.abb.1001027
Copyright License: © 2021 Mantareva V, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Zn(II) phthalocyanine; Mestranol; Singlet oxygen; Cholesterol photosensitized oxidation; Photodynamic therapy
Mestranol moieties clicked to Zn(II)phthalocyanine for controllable photosensitized oxidation of cholesterol
Vanya Mantareva1*, Meliha Syuleyman1,2, Adriana Slavova-Kazakova1, Ivan Angelov1 and Mahmut Durmus2
1Institute of Organic Chemistry, Centre of Phytochemistry, Bulgarian Academy of Sciences, Acad. G. Bonchev str, Bld. 9, 1113 Sofia, Bulgaria
2Department of Chemistry, Gebze Technical University, P.O. Box 141, Gebze 41400, Kocaeli, Turkey
*Address for Correspondence: Vanya Mantareva, Institute of Organic Chemistry, Centre of Phytochemistry, Bulgarian Academy of Sciences, Acad. G. Bonchev str, Bld. 9, 1113 Sofia, Bulgaria, Email: [email protected]; [email protected]
Four mestranol moieties were chemically linked to Zn(II) phthalocyanine (4) by cycloaddition “Click” reaction using a tetra-azidoethoxy substituted Zn(II)-phthalocyanine (3). The alkyl-azido coupling reaction was realized between azido groups of 3 and alkyl group of mestranol. The alkylation reaction was carried out to obtain cationic Zn(II) phthalocyanine derivative (5). The new compounds were chemically characterized by the known analytical methods. The absorption and fluorescence properties were studied in comparison. The absorption maxima of phthalocyanines 3, 4 and 5 were recorded at approx. shifts of 8 - 12 nm in the far- red region (680 - 684 nm) and the fluorescence maxima (692 - 693 nm) as compared to unsubstituted ZnPc (672 nm, 680 nm) in DMSO. The studies of singlet oxygen generation of 3, 4 and 5 showed relatively high values such as 0.52 for 3; 0.51 for 4 and 0.46 for 5. The fluorescence lifetime of 3.15 ns (3), 3.25 ns (4) and 3.46 ns (5) were determined with lower than the value than for the used standard ZnPc (3.99 ns). The high photo stability was observed for compounds 3, 4 and 5. In addition, the photosensitized oxidation of cholesterol was compared for 3 and 4 with much lower values of oxidation potential than for unsubstituted ZnPc which suggests that the substitution groups influenced on the photooxidation index of the target molecule.
The well-known advances of the photodynamic therapy (PDT) as a curative method with many biomedical applications have been accepted for other applications [1-4]. Briefly, the PDT routine involves a non-toxic in the dark photosensitizer (PS) which after precise light exposure initiates the photophysical conversions of molecule resulting in generation of singlet oxygen and other reactive oxygen species (ROS). Both mechanisms of photosensitization (by energy or by electron/proton transfer from the excited triplet state of PS) lead to chain reactions which maintain the generation of highly reacting species that incite the cell destruction by photosensitized oxidation [5,6]. Presently the PDT has been evaluated as an alternative therapeutic approach to combat the health problem of fast development of drug resistance because it can cause the irreversible damages of the natural biomolecules as cellular ingredients [7].
The structural characteristics of photosensitizers for PDT are known as critical for their photophysical properties and interactions with disordered cells [8]. The charge and hydrophobicity and the structural steric hindrance of the PS’s molecule must be considering for a better membrane penetration which is of importance for the effective photo-destruction of the cells in the vicinity of irradiation. The up-to-date knowledge about the right structure for PDT sensitizers suggested that positively charged compounds are more favorable than anionic and non-charged for usage as PSs. This is because of the more sufficient electrostatic interaction with the membranes. Moreover, the PS molecules tend to interact with cell membranes through the peripheral substituents linked to the PS molecule and the substituents can also facilitate the PS binding to the cellular surface (Sun, et al. 2019). In consideration to the cell’s ingredients, it is well agreed that the molecules among the group of lipids and steroids are in high occurrence in the membranes with their characteristic of classical membrane anchors [9]. Moreover, their biological functions to stimulate the PS binding to membranes by receptor recognition of the natural steroids in the disordered cells [10].
The natural sterols occur in the membranes of yeasts, along with the plants and animals, and have a basic function of architectural fragments in cells’ growing [11]. The studies suggested that cholesterol cannot be replaced by the native ergosterol typical for fungus, and it must be small amount of endogenous cholesterol [12]. There is much evidence that sterols are important for the physical properties of the membrane by controlling the rigidity which is expressed as membrane’s fluidity and its cell defense function. There are more than a hundred sterols in the living organisms where cholesterol and its homologs occur in cell membranes which are well known targets of oxidative reactions (Zhao, et al. 2020).
Phthalocyanines (Pcs) have been intensively studied over the past two decades as second-generation photosensitizers for various PDT usages [13]. Although the great number of newly synthesized substituted Pc complexes (MPcs) with advanced physicochemical and spectroscopic properties appear, only a few numbers of them have passed the regulation for clinical applications (Li, et al. 2019). The research and development of novel Pcs based photosensitizers with photodynamic activity focus on addition of some functional groups in advance to the targets such as tumor cells or pathogenic microbes [2]. One possible approach is the linking of biologically active and cell-specific compounds to Pc ring. For example, the bioconjugation of Ge(IV)Pc to cholesterol moieties facilitates the Pc transport by favorable association with low-density lipoproteins (LDL) which further favorable interaction with membrane cell receptors to potentiate localization for increasing the PDT efficacy [14]. As is well known the plasma membrane is frequently the principal residence of sterols. The most prokaryotic membranes are bilayers containing phospholipid and protein where sterols are situated in each monolayer in a non-homogeneous distribution or in rare cases, they can undergo re-distribution between the monolayers (so called “flip-flop”). The limited number of studies present the photosensitizer’s molecule linked to mestranol or other steroids in respect to photo- physicochemical properties and photooxidation potential [15].
The study presents new conjugates of Zn(II) phthalo-cyanine linked to mestranol (4) by a cycloaddition (“click”) reaction and the cationic derivative 5 as prepared after the methylation. The phthalocyanines 3, 4 and 5 were investigated in comparison to ZnPc for the main light associated properties of absorption, fluorescence and singlet oxygen generation that are referring to photosensitization process and the PDT application. The role of the azidoethoxy substitution groups to ZnPc ring for 3 and the mestranol substitution via triazole ring for 4 was evaluated versus ZnPc in the photosensitized oxidation of cholesterol. The quaternization to 5 has a slight effect on the main physicochemical properties.
Chemicals
The solvents used for synthesis and purification such as dimethylformamide (DMF), dichloromethane (DCM), tetrahydrofuran (THF) and n-pentanol were used after additional treating [16]. The solids such as anhydrous potassium carbonate and zinc(II) acetate dihydrate were dried on glass oven before used. Unsubstituted zinc phthalocyanine, 1,8-diazabicyclo [5.4.0] undec-7-ene (DBU), 4-iodomethane and 2-bromoethanol were purchased from Aldrich (FOT, BG). Purification was performed by flash chromatography on silica 60 Å (0.04–0.63 mm) and Bio-Beads S-X1 (200–400 mesh) from Bio-Rad. The starting compounds 4-nitrophthalonitrile, 2-azidoethanol (1) and 4-(2-azidoethoxy) phthalonitrile (2) as shown in Scheme 1, were prepared following similar procedure [23]. The used as a singlet oxygen scavenger 1,3-Diphenylisobenzofuran (DPBF) was purchased from Fluka Analytical (Labimex, BG). Cholesterol as an anhydrous powder was ordered from Sigma (FOT, Bulgaria).
Synthesis
Synthesis of 2(3),9(10),16(17),23(24)-Tetrakis 2-(azi-doethoxy) zinc(II) phthalocyanine (3): The lithium cuttings were added to 2 mL dry n-pentanol, flushed with argon and then the stirring continued at 60 °C until lithium was fully dissolved. The solution was cooled to room temperature and 134 mg (0.582 mmol) 4-(2-azidoethoxy) phthalonitrile (2) was added. Then the reaction mixture was heated to 120 °C, under argon for 2 h followed by the lowering of temperature to 80 °C. The salt of Zn(II) acetate dihydrate (2.33 mmol) was added and flushed with argon. The reaction mixture was stirred for additional 2 h and after cooling the product was isolated by addition to hexane. The obtained fine greenish sediment was washed several times and isolated by centrifugation. The column chromatography was carried out on silica gel by eluent THF: Hexane (3:1). Yield: 40 mg (30 %); C40H28N20O4Zn; M. w.: 918.19 g/mol; FT-IR (KBr): ν, cm-1: 3066 (Ar -CH), 2927-2856 (aliph. -CH), 2107 (N3), 1609 (C=C), 1123-1041 (C-O-C), 956, 858, 745, 649. 1H-ЯМР (d6-DMSO), δ, ppm: 4.04-4.03 (m, 8H, CH alip), 4.72-4.71 (br, 8H, CH alip), 7.35-7.31 (m, 1H, CH arom), 7.61-7.51 (m, 4H, CH arom), 8.38-8.27 (m, 4H, CH arom), 8.84-8.75 (m, 3H, CH arom); UV-Vis (DMSO): λmax, nm (log ɛ): 680 (5.42); 614 (4.77); 353 (5.07). MALDI-TOF-MS m/z: MALDI-TOF (+): 915 [M -2H]+, 887 [M-2N -2H]+; 861 [M-4N -H]+.
Synthesis of 2(3),9(10),16(17),23(24)-Tetrakis (mes-tranolethoxy) zinc(II) phthalocyanine (4): Compound 3 (41 mg, 0.045 mmol) was mixed with mestranol (69.8 mg, 0.225 mmol), CuSO4. 5H2O (56 mg, 0.225 mmol) and sodium ascorbate (90 mg, 0.45 mmol) in the solvents DMF/DCM and H2O (1:1:1) and the reaction mixture was stirred at room temperature for 36 h under argon. The product was extracted with DCM - water and then purified by column chromatography using THF: Hexane (3:1) as an eluent. Yield: 22 mg (23%); C124H132N20O12Zn; M.w.: 2159.91 g/mol; IR (ATR) ν (cm-1): 2952 - 2854 (Aliph. C-H), 1722(s) (Ald. C=O), 1609, 1577 (Arom. C=C), 1256, 1034 (C-O-C), 919, 866, 843, 702. UV-Vis (DMSO): λmax, nm (log ɛ): 684 (4.76); 615 (4.16); 350 (5.05). MALDI-TOF-MS m/z: 2163.318 [M+3H]+.
Synthesis of 2(3),9(10),16(17),23(24)-Tetrakis met-hoxy(mestranolethoxy) zinc(II) phthalocyanine (5): Compound 4 (30 mg, 0.014 mmol) was dissolved in dry DMF (2 mL) and an excess of methyl iodide (12 mg, 0.084 mmol) was added. The reaction mixture was stirred at room temperature under argon for 24 hours. The product was purified by chromatography using a mixture of DCM and ethanol (10:1) and then was dried in vacuum. Yield: 24 mg (62%); C128H144I4N20O12Zn; M.w. 2727.69 g/mol; IR (ATR) ν (cm-1): 2345, 2278, 1712, 1472, 1460, 1403, 1337-1232, 1094-1016 (C-O-C), 879, 854, 746, 668. UV-Vis (DMSO): λmax, nm (log ɛ): 681 (4.92); 614 (4.23); 340 (4.81). MALDI-TOF-MS m/z: 560.200 [M-4I]4+.
Photophysical and photochemical measurements
The experimental part for the physicochemical properties of phthalocyanines 3, 4 and 5 is described in Supplementary material.
Cholesterol photosensitized oxidation
The freshly prepared reaction mixtures contained the known amounts of the studied photosensitizer (3, 4 and ZnPc) and cholesterol with concentration 0.05 M, all placed in 10 ml glass vials wrapped with Al-foil. The aliquots were taken from phthalocyanines stock solutions in dimethyl sulfoxide (DMSO) and added to a cholesterol solution in dichloromethane (CH2Cl2). The procedure was adjusted to study a photosensitizer which produced singlet oxygen (type II mechanism) as the main oxidative reagent in the studied lipid oxidation [15]. Three types of controls were prepared: (1) sensitizer-free samples without phthalocyanine and light, (2) the samples irradiated with the same light dose as the photosensitized samples and (3) the dark control samples containing the studied phthalocyanine without irradiation. Irradiations of the experimental samples were performed with light emitting diode (LED) at 635 nm or with 365 nm light after an incubation period of 5 min. The range of light doses of 8, 17, 25, 34 and 42 J. cm-2 with a power density of 30 mW.cm-2 were applied. All experiments were carried out in triplicates with varying the phthalocyanine’s concentration. Lipid hydroperoxides (ChlOOH) were determined according to a micro-method which is based on the iodometric determination of peroxide value (PV) (Moringer, et al. 1995). The results of PV accumulation were plotted. The results are presented based on three independent experiments with standard deviations. The obtained curves for peroxide accumulation are conveyed as M.s-1.
Three Zn(II) phthalocyanines, one substituted with four azidoethoxy groups (3) and the next linked to four mestranol moieties by 1,2,3- triazole fused ring (4) and the cationic derivative obtained with methyl iodide (5) were studied with relatively high values of the photo- physicochemical properties. The photosensitized oxidation against cholesterol of the substituted ZnPc3 and 4 was with much lower values than for the unsubstituted ZnPc. Considering the fluorescence, singlet oxygen and photooxidation potential of mestranol conjugated ZnPcs, both features as promising photosensitizers for PDT applications.
Synthesis
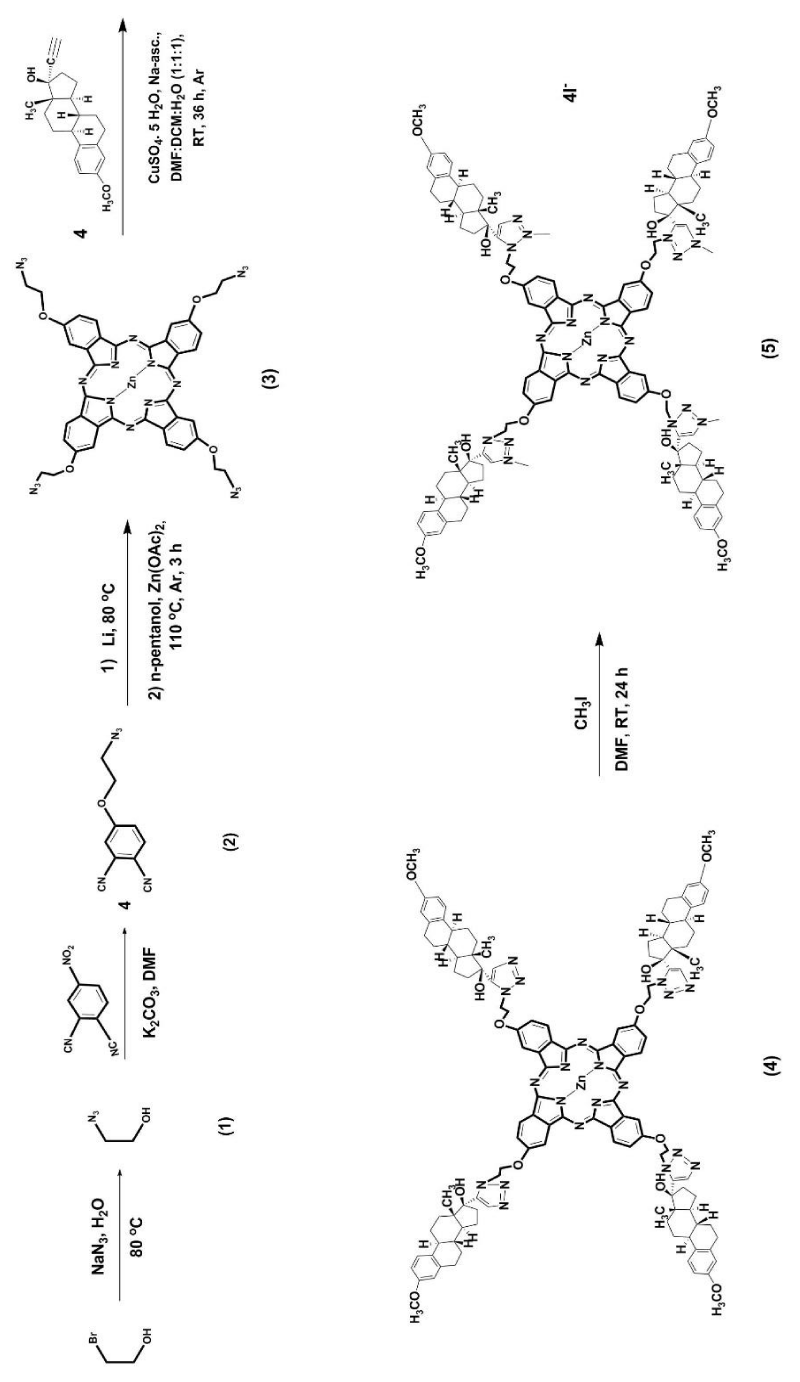
Scheme 1 shows the used five steps synthetic route for preparation of a novel Zn(II) phthalocyanine linked to mestranol (4) and its quaternized derivative (5) bearing four cationic charges, one on each triazole fused ring. In this study, a phthalonitrile precursor of 4-(2-azidoethoxy) phthalonitrile (2) was synthesized from the commercial 2-bromoethanol which was converted to 2-azido ethanol according to the literature procedure [16]. The type SNAr substitution reaction of compound (1) with 4-nitrophthalonitrile was carried out [17]. This includes the presence of K2CO3 as a base, in dry DMF at 40 °C under argon to obtain compound (2) in high yield and purity. The target tetra-azidoethoxy-substituted Zn(II) phthalocyanine (3) was synthesized from dinitrile (2) in n-pentanol, drops DBU, 110 °C, under argon. The cyclotetramerization started by addition of lithium cuttings, dissolved in dry n-pentanol in the beginning of reaction. Then, zinc(II) acetate dihydrate was added to obtain the complex compound (3) for a total reaction time of 5 hours. The linkage between azide groups of tetra- azidoethoxy- Zn(II) phthalocyanine (3) and alkyne group of mestranol was carried out following the coupling reaction. The reaction developed by Rоlf Huisgen known as “Click” is an azide-alkyne or 1,3-dipolar addition reaction and it was recently introduced in phthalocyanine synthesis [18]. The applied reaction conditions of 1 eqv. CuSO4. 5H2O and 6 eqv. Sodium ascorbate in a mixture of DMF/DCM: H2O (1:1:1) at room temperature are appropriated. The covalent linkage through a 1,2,3-triazole-fused heterocyclic ring of compound (4) appears suitable for the next reaction of quaternization. The reaction was performed with an excess of methyliodide in dry DMF at room temperature (24 h) to give the cationic compound (5). The new phthalocyanines were purified by flash chromatography on silica gel with an eluent mixture of THF: Hex (3: 1) or on Bio-beads SX-3 beads and DCM as eluent. The structures of all novel compounds were analyzed by the FT-IR, 1H NMR, MALDI-TOF mass and UV-Vis spectroscopic techniques.
Scheme 1: Synthetic pathway for substitution of Zn(II) phthalocyanine (3) with mestranol moieties (4) via Cu(I) catalyzed azide-alkyne cycloaddition and its cationic derivative (5).
2-Azidoethoxy substituted Zn(II) phthalocyanine (3) was identified by disappearance of a peak at 2230 cm-1 for C≡N stretching peak in the FT-IR spectra of the phthalonitrile derivative (2) in Scheme 1. The peaks of the aromatic CH at 3066 cm-1 and for the aliphatic CH 2927-2856 cm-1 were observed. The formation of phthalocyanine-mestranol conjugate (4) was confirmed by the disappearance of N3 stretching peak at 2107 cm-1 in the FT-IR spectrum. Also, the aromatic CH and aliphatic CH stretching peaks were observed at 2952 - 2854 cm-1 and 1722(s), 1609, 1577 cm-1, respectively in the FT-IR. The quaternized derivative 5 showed signals of aromatic CH at 3165, 3019 cm-1 and for aliphatic CH at 2972-2754 cm-1. The mass spectra of the studied phthalocyanines were obtained using dithranol (DIT) as MALDI matrix. The molecular ion peaks were observed at m/z: 915.87 [M]+ for phthalocyanine 3, m/z: 2163.318 [M+3H]+ for phthalocyanine 4, as m/z: 560.200 [M-4I]4+ for phthalocyanine 5 in their MALDI-TOF mass spectra. The 1H-NMR spectra of the compounds despite the broad peaks were all detected at the aromatic region of the spectra. The structure of 2(3), 9(10), 16(17), 23(24) - tetrakis-(2-azidoethoxy) phthalocyaninato) zinc(II) (3) was confirmed by 1H-NMR spectra. The signals appear between 4.04 ppm and 8.84 ppm. The protons of the substituents and the phthalocyanine ring were observed in their expected regions. 1H-NMR spectra of compounds 4 and 5 after, both were difficult for description. Cupper has characteristic to mask the signals, or because of the aggregation of both Zn(II)-phthalocyanine bioconjugates with mestranol moieties.
Absorption and fluorescence properties
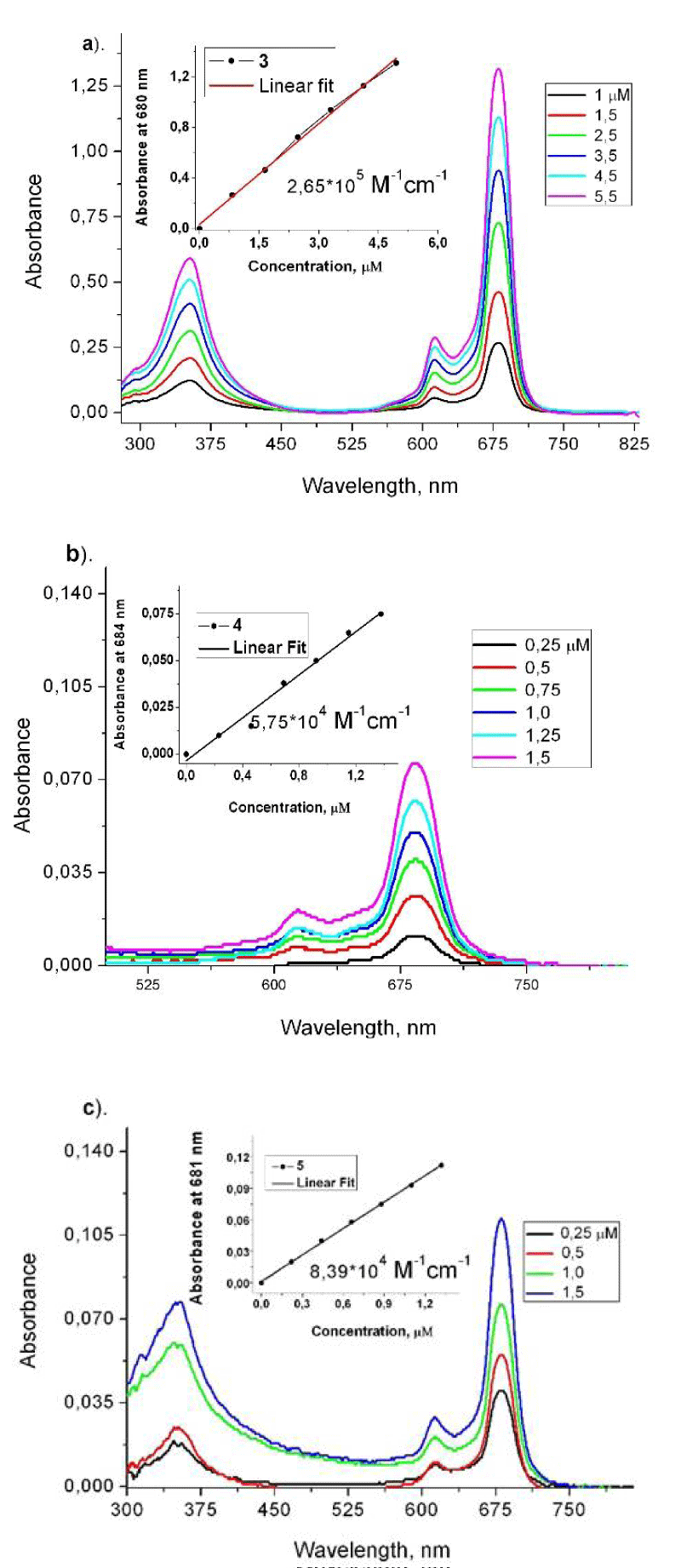
The absorption spectra of the starting tetra- azidoethoxy substituted Zn(II)-phthalocyanine 3 and the both conjugates with mestranol 4 and the quaternized derivative 5 were studied in DMSO solutions for different concentrations (Figure 1).
Figure 1: Absorption spectra of phthalocyanines 3, 4 and 5 in DMSO solutions with different concentrations. Inset: O.D. at Q-band max for a concentration range.
The phthalocyanines 3, 4 and 5 showed sharp Q band of absorptions at 680 nm, 684 nm and 681 nm respectively. The new phthalocyanines showed the bathochromic shifted absorbances as compared to the basic ZnPc molecule (672 nm). The characteristic B band (Soret) showed a broad spectrum between 330-365 nm (Figure 1a). The Soret bands appear at 340 nm (3), 350 nm (4) and 353 nm (5) which is typical for Pc compounds. The absorption spectra were recorded at different dye concentrations in DMSO solution (Figure 1) to calculate the values for extinction coefficients (e), which values are inserted in figure 1 for the Q-bands. The obtained spectra for the studied concentration range showed monomeric state of compounds and according to the Beer-Lambert-Bouguer law in DMSO solutions [19].
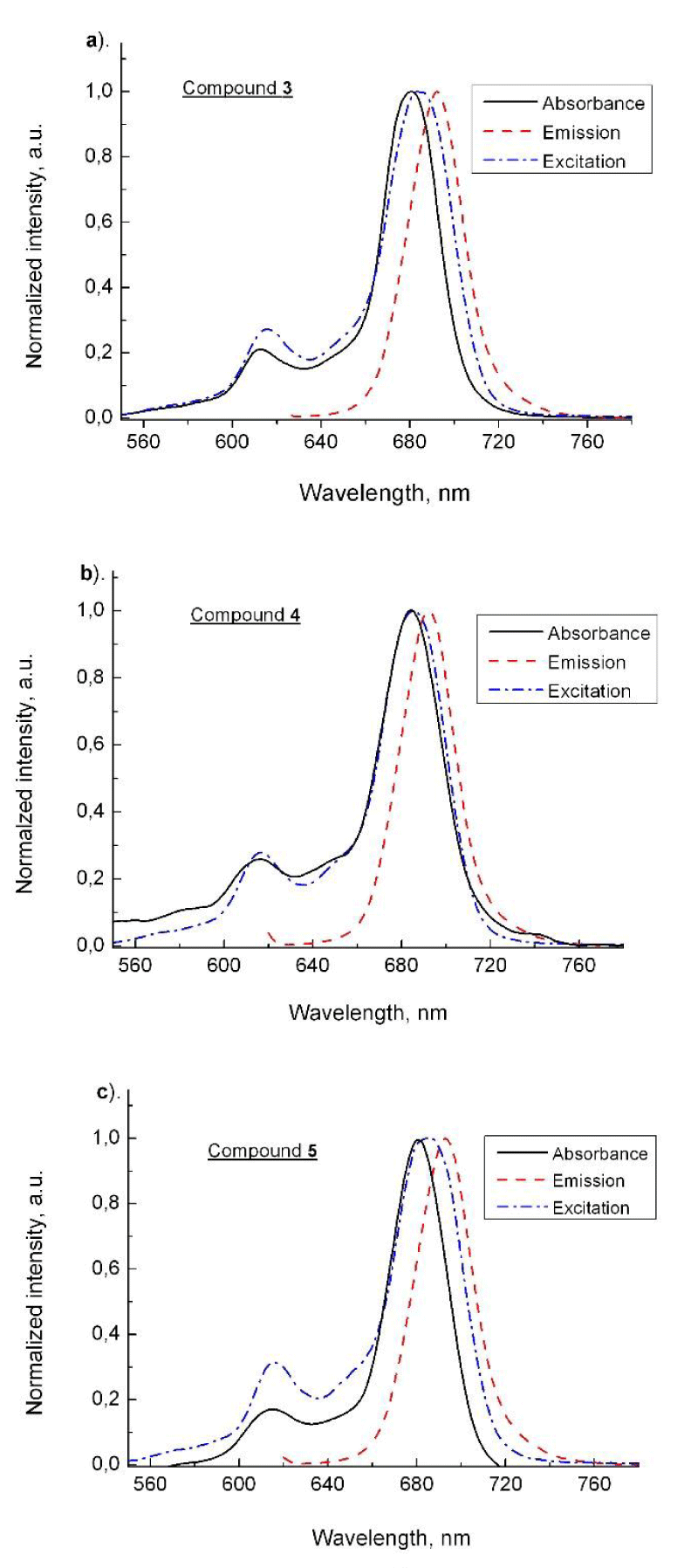
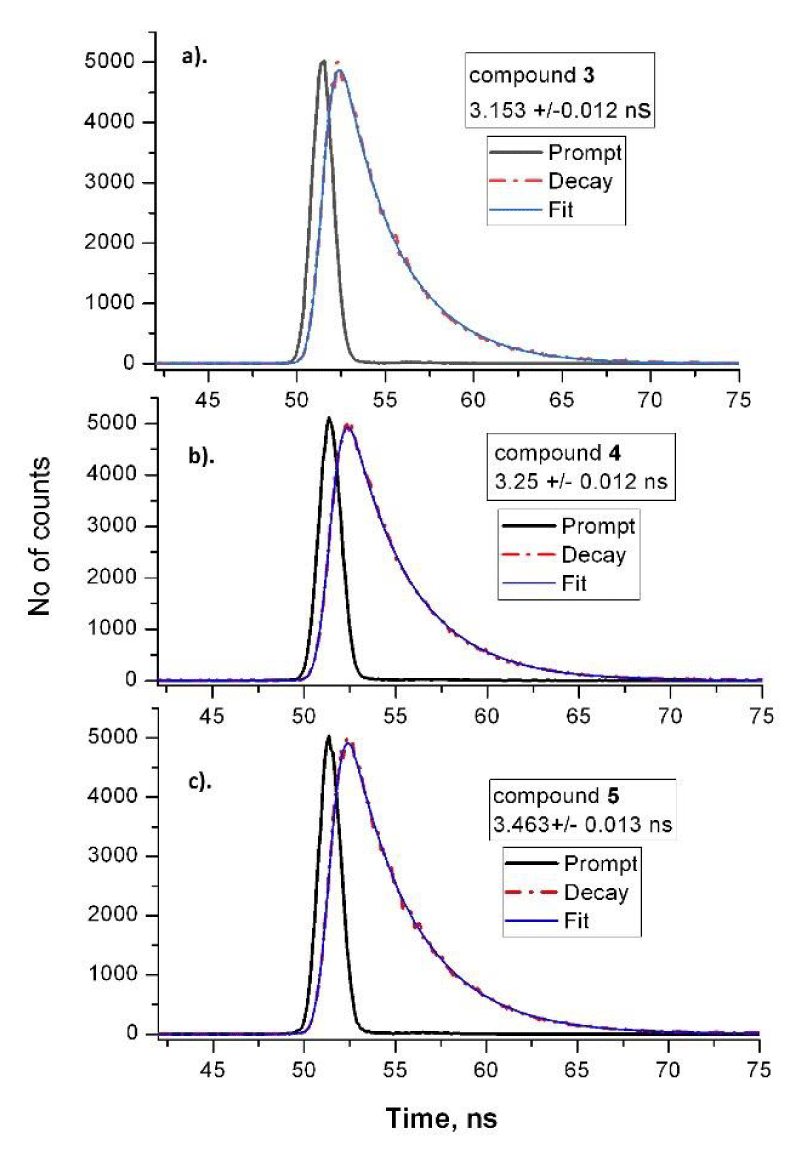
The fluorescence spectra of phthalocyanine (3) and the conjugates (4 and 5) showed red shifted maxima at approx. 12 nm as compared to their absorbances (Figure 2a-c). The emission maxima of the new compounds were recorder at 692 nm (3), 693 nm (4) and 693 nm (5) respectively at excitation wavelength at 610 nm. Figure 2 presents the excitation spectra which as seen are in agreement to the absorbances which is suggesting the uniform molecular solutions for three studied phthalocyanines. The time-resolved fluorescence study was carried out at excitations at 360, 610 and 660 nm based on the time-correlated single photon counting method. As seen the mono-exponential curves for the studied phthalocyanines 3, 4 and 5 were obtained (Figure 3). This suggests that the fluorescence belongs to the solutions with structurally one and the same molecular species. The obtained fluorescence lifetimes have the relatively similar values (3.15, 3,25 and 3.46 ns) but lower than the same parameter for the basic molecule (ZnPc, 3.99 ns). This observation often happens for differently substituted phthalocyanines which were measured with high or low values of the singlet excited state properties in dependence on the nature of the substitution groups [20]. The fluorescence lifetime for azidoethoxy- substituents in compound 3 (3.15 ns) showed slightly lower value than for 4 (3.25 ns) and for quaternized derivative 5 (3.46 ns). The obtained promising values of the properties of the singlet state and especially for the cationic conjugate 5 suggest that the photophysical properties of the studied phthalocyanines are suitable for the PDT usage.
Figure 2: Fluorescence emission, excitation and absorption spectra of phthalocyanines 3 (a), 4 (b) and 5 (c) presented in normalized intensity.
Figure 3: Time-resolved fluorescence monoexponential curves of phthalocyanines 3 (a), 4 (b) and 5 (c) obtained in DMSO at excitation with 674 nm laser.
Singlet oxygen generation
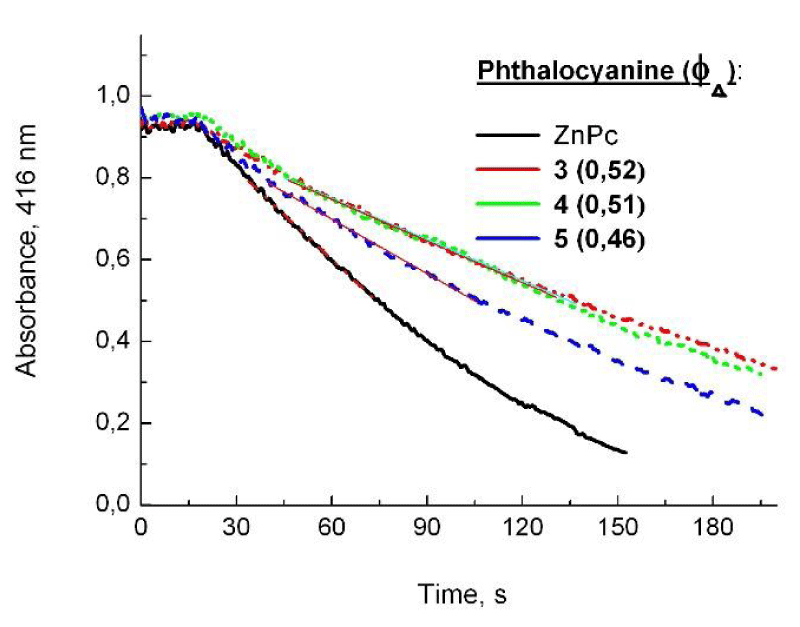
The main cytotoxic product of photosensitization in the presence of phthalocyanines is assumed to be singlet oxygen [21]. The experiments which are concerning evaluation of singlet oxygen production rate for the new phthalocyanines were carried out by indirect chemical method with a compound (DPBF) which is a singlet oxygen scavenger. The speed of the photooxidation of molecules was evaluated by signal from the absorption maximum at 416 nm of DPBF (Figure 4).
Figure 4: Absorbances of DPBF recorded during the time of LED 635 nm irradiation of phthalocyanines 3, 4 and 5 towards unsubstituted ZnPc (0.67) in DMSO.< 0.05).
The control of reaction was performed by recording the absorbances of the studied phthalocyanines (3, 4 and 5) before and after irradiation. The results suggested that the applied irradiation did not decompose the studied photosensitizers. The spectra of the DPBF solutions with or without light were also recorded for control the photobleaching of DPBF because of generated singlet oxygen [22]. The starting phthalocyanine 3 and the conjugates 4 and 5 showed singlet oxygen generation in DMSO of 0.52 (3), 0.51 (4) and 0.46 (5) calculated from the speed of decrease of DPBF absorbance during LED 635 nm irradiation (Figure 4). The singlet oxygen quantum yield of phthalocyanine 3 was found a bit higher than for the conjugate 4 and 5 maybe due to some physical quenching due to substituents [23].
Photosensitized oxidation of cholesterol
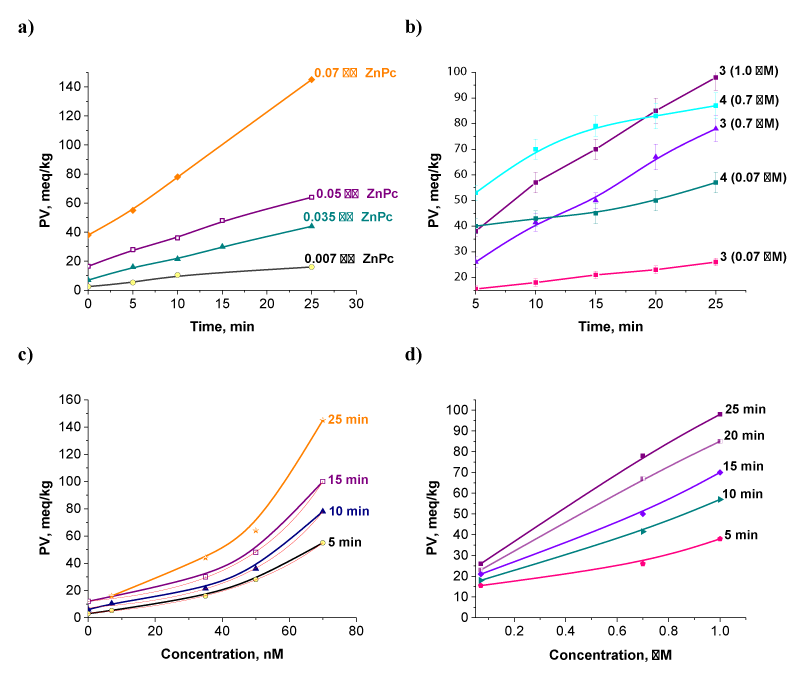
The generation of cholesterol hydroperoxides (ChlOOH) because of the photosensitized oxidation of cholesterol in the presence of phthalocyanines 3, 4 or ZnPc and the irradiation with LED 635 nm were determined (Figure 5a,b). As can be seen there is an accumulation of hydroperoxides which rise with the light dose increasement. The effect of higher concentrations of ZnPc (up to 70 nM) in a range of light doses was not significant for the cholesterol oxidation process. Thus, can be explained with the initial accumulation of the primary oxidation products, ChlOOH during the studied period of time. It is well documented that phthalocyanines as singlet oxygen photosensitizers provoke the photosensitized oxidation, which is mediated predominantly by the excited singlet oxygen (1O2) i.e. type II mechanism. Girotti, [24] described the reaction as the followed equation:Cholesterol + O2 + hν + sensitizer→ [1O2] → 5α-OOH + 6α-OOH
The concentration range was chosen so that to have the oxidation products because of the photosensitized oxidation. The obtained kinetic curves presented in figure 5a,b show a liner accumulation of hydroperoxides with the increment of the light dose. Increasing the concentration of ZnPc at doses up to 0.07 μM has no effect on the cholesterol oxidation process, i.e. of the accumulation of the primary oxidation products (5α-OOH and 6α-OOH), as a function of reaction time and light dose respectively. The concentration at which the linearity has being kept in presence of the new synthesized compound 3 reaches up to 1.0 μM. This is not the case with the compound 4. Nevertheless, changes in oxidation rates occur in all cases of photosensitized oxidation. Obviously, the oxidation rate depends on the structure of the sensitizer on the one hand and its concentration on the other.
The values obtained for the oxidation rates in presence of studied sensitizers at different concentrations are presented in table 1. It is seen from the results obtained that both studied new compounds 3 and 4 have almost equal oxidation rates (≈ 4.3 x 10-6) at 0.07 μM while the starting ZnPc ensures 8-fold higher rate (36.1 x 10-6) at the same concentration. The 10-fold increase in the concentration of 3 (from 0.07 μM to 0.7 μM) leads to 4-fold higher oxidation rate. For comparison, the same increase in the concentration but with an order of magnitude lower values (0.007 - 0.07 μM) in case of ZnPc ensures 8-fold higher rate of the oxidation process. In table 1 are shown the calculated values of the oxidation rates in the presence of the studied phthalocyanines for a range of concentrations. The obtained results suggest that both substituted phthalocyanines 3 and 4 have almost equal oxidation rates of 4.3 x 10-6 at 0.07 μM while the unsubstituted ZnPc reacted much stronger with rate of 36.1 x 10-6 at the same concentration. The obtained dependences between concentration of phthalocyanines and that of ChOOH are presented in figure 5c,d. As seen in concentration range between 0.07 μM and 1.0 μM of compound 3 the curves go linear dependence for all the studied time intervals (Figure 5d). In case of ZnPc the levels of the oxidation products (ChOOH) increase following an exponential curves at concentration up to 0.07 μM (Figure 5c).
| Table 1: Oxidation rates in the presence of phthalocyanines with different concentrations. | ||
| Photosensitizer | Concentration, μM |
Oxidation rate R, M.s-1 (x 106) |
| ZnPc 3 | 0.07 | 4.3 |
| 0.7 | 21.5 | |
| 1.0 | 25.5 | |
| ZnPc 4 | 0.07 | 4.2 |
| 0.7 | n.d. | |
| ZnPc | 0.007 | 4.5 |
| 0.035 | 12.2 | |
| 0.05 | 15.8 | |
| 0.07 | 36.1 | |
The results suggested that that cholesterol undergoes much faster photosensitized oxidation in the presence of unsubstituted ZnPc than after substitution with azidoethoxy- group for compounds 3 and mestranol units for compound 4. Maybe the cholesterol oxidation in mild speed is preferable to control the effect during PDT as the main target biomolecule [25]. These observations can be used to ensure the cure results than the harsh oxidation process provoked by the sensitizer administered.
Figure 5: Kinetics of peroxide accumulation during the photosensitized oxidation with ZnPc (a) and phthalocyanine 3 and 4 (b) after cholesterol irradiation with red light (LED 635 nm) for 25 min and the functional dependences on the concentration during irradiations for different time intervals (c and d).
Bioconjugates of Zn(II)-phthalocyanines with mestranol and the cationic derivative were synthesized on the basis of Zn(II)-phthalocyanine with four azidoethoxy groups (3). The substitution of 3 was successfully proceeded by Click reaction resulting to phthalocyanine 4. The quaternization of the linker group of triazole ring was realized by one methyl group per triazole unit. The phthalocyanines 3, 4 and 5 were studied with physicochemical properties showing the characteristic red shifts of the fluorescence maxima to the far-red region and the relatively high singlet oxygen generation upon red-light exposure. The physicochemical properties together with cholesterol photosensitized oxidation showed the promising potential of the mestranol linked ZnPc as photosensitizer. In addition, the target molecule of cholesterol was studied for the photooxidation ability in the presence of 3 and 4 as compared to the ring molecule of ZnPc. The results suggest that the addition of substituents improved the physicochemical properties of ZnPc molecule and can lead to gentle and more controllable oxidation potential of the PDT routine.
Summary
The study involves investigations concerning the new photosensitizers for application the photodynamic therapy. Three new phthalocyanines were synthesized as tetra- substituted Zn(II)-phthalocyanine complexes and the main physicochemical properties the new phthalocyanines were studied. The oxidation potential of the phthalocyanine complexes for photosensitized oxidation of cholesterol as the main target molecule in the cell membranes were studied.
Thanks to the National Science Fund of Bulgaria for the project KP-06-H29/11, 2018.
- Ben-Hur E, Green M, Prager A, Kol R, Rosenthal I. Phthalocyanine photosensitization of mammalian cells: biochemical and ultrastructural effects. Photochem Photobiol. 1987; 46: 651-656.
- Galstyan A. Turning photons into drugs: phthalocyanine-based photosensitizers as efficient photoantimicrobials. Chemistry. 2021; 27: 1903-1920. PubMed: https://pubmed.ncbi.nlm.nih.gov/32677718/
- Nakonieczna J, Wozniak A, Pieranski M, Rapacka-Zdonczyk A, Ogonowska P, et al. Photoinactivation of ESKAPE pathogens: overview of novel therapeutic strategy. Future Med Chem. 2019; 11: 443-461. PubMed: https://pubmed.ncbi.nlm.nih.gov/30901231/
- MacDonald IJ, Dougherty TJ. Basic principles of photodynamic therapy. J Porphyrins Phthalocyanines. 2001; 5: 105-129.
- Bacellar IOL, Tsubone TM, Pavani C, Baptista MC. Photodynamic Efficacy: From Molecular Photochemistry to Cell Death. Int J Mol Sci. 2015; 16: 20523-20559. PubMed: https://pubmed.ncbi.nlm.nih.gov/26334268/
- Bin ZS, Awlad HM, Rachel N, Varshil M, Taib MK, et al. A review on antibiotic resistance: alarm bells are ringing. Cureus. 2017; 6: 1403. PubMed: https://pubmed.ncbi.nlm.nih.gov/28852600/
- Paronyan MH, Koloyan HO, Avetisyan SV, Aganyants HA, Hovsepyan AS. Study of the possible development of bacterial resistance to photodynamic inactivation. Biolog J Armenia. 2019; 1: 17-22.
- Li X, Zheng BD, Peng XH, Li SZ, Ying JW, et al. Phthalocyanines as medicinal photosensitizers: Developments in the last five years. Coord Chem Rev. 2017; 379: 147-160.
- Albuquerque HMT, Santos CMM, Silva AMS. Cholesterol-Based Compounds: Recent Advances in Synthesis and Applications. Molecules. 2019; 24: 116. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6337470/
- El-Akra N, Noirot A, Faye JC, Souchard JP. Synthesis of estradiol-pheophorbide a conjugates: evidence of nuclear targeting, DNA damage and photodynamic activity in human breast cancer and vascular endothelial cells. Photochem Photobiol Sci. 2006; 5: 996-999. PubMed: https://pubmed.ncbi.nlm.nih.gov/17077894/
- Girotti AW, Korytowski W. Cholesterol Peroxidation as a Special Type of Lipid Oxidation in Photodynamic Systems. Photochem. Photobiol. 2019; 95: 73-82. PubMed: https://pubmed.ncbi.nlm.nih.gov/29962109/
- Khan EH, Ali H, Tian H, Rousseau J, Tessier G, et al. Synthesis and Biological Activities of Phthalocyanine-Estradiol Conjugates. Bioorg Med Chem Lett. 2003; 13: 1287-1290. PubMed: https://pubmed.ncbi.nlm.nih.gov/12657265/
- Segalla A, Milanesi C, Jori G, Capraro HG, Isele U, et al. CGP 55398, a liposomal Ge(IV) phthalocyanine bearing two axially ligated cholesterol moieties: a new potential agent for photodynamic therapy of tumors. Br J Cancer. 1994; 69: 817-825. PubMed: https://pubmed.ncbi.nlm.nih.gov/8180009/
- Orekhov PS, Kholina EG, Bozdaganyan ME, Nesterenko AM, Kovalenko IB, et al. Molecular mechanism of Uptake of Cationic Photoantimicrobial Phthalocyanine across Bacterial Membranes Revealed by Molecular Dynamic Simulations. J Phys Chem. 2018; 122: 3711-3722. PubMed: https://pubmed.ncbi.nlm.nih.gov/29553736/
- Girotti AW, Korytowski W. Cholesterol as a Singlet Oxygen Detector in Biological Systems, In: Methods in Enzymology, Academic Press. 2000; 319: 85-100. PubMed: https://pubmed.ncbi.nlm.nih.gov/10907502/
- Zorlu Y, Un I, Hirel C, Dumoulin F, Ahsen V. Phthalonitriles Functionalized for Click Chemistry. Design, synthesis and Structural Characterization. J Chem Crystallogr. 2013; 43: 636-645.
- George RD, Snow AW. J Heterocyclic Chem. 1995; 32: 495-498.
- Novakova V, Zimcik P, Miletin M, Kopecky K, Ivincova J. A phthalocyanine-mestranol conjugate for photodynamic therapy prepared via click chemistry. Tetrahedron Lett. 2010; 51: 1016-1018.
- Jia X, Yang FF, Li J, Liu JY, Xue JP. Synthesis and in Vitro Photodynamic Activity of Oligomeric Ethylene Glycol–Quinoline Substituted Zinc(II) Phthalocyanine Derivatives. J Med Chem. 2013; 56: 5797-5805. PubMed: https://pubmed.ncbi.nlm.nih.gov/23786380/
- Cakir V, Goksel M, Durmuş M, Biyiklioglu Z. Synthesis and photophysicochemical properties of novel water soluble phthalocyanines. Dyes Pigm. 2016; 125: 414-425.
- Ekineker G, Goksel M. Synthesis of both peripheral and non-peripheral substituted metal-free phthalocyanines and characterization. Tetrahedron. 2020; 76: 130878.
- Nishie H, Kataoka H, Yano S, Kikuchi JI, Hayashi N, et al. A next-generation bifunctional photosensitizer with improved water-solubility for photodynamic therapy and diagnosis. Oncotarget. 2016; 7: 74259-74268. PubMed: https://pubmed.ncbi.nlm.nih.gov/27708235/
- Simpson CA, Huffman BJ, Gerdon AE, Cliffel DE. Unexpected Toxicity of Monolayer Protected Gold Clusters Eliminated by PEG-Thiol Place Exchange Reactions. Chem Res Toxicol. 2010; 23: 1608-1616. PubMed: https://pubmed.ncbi.nlm.nih.gov/20715858/
- Girotti AW. Photosensitized oxidation of membrane lipids: reaction pathway, cytotoxic effects, and cytoprotective mechanisms. J Photochem Photobiol B Biol. 2001; 63: 103-113. PubMed: https://pubmed.ncbi.nlm.nih.gov/11684457/
- Jia HR, Zhu YX, Chen Z, Wu FG. Cholesterol-Assisted Bacterial Cell Surface Engineering for Photodynamic Inactivation of Gram-Positive and Gram-Negative Bacteria. ACS Appl. Mater Interfaces. 201; 9: 15943-15951. PubMed: https://pubmed.ncbi.nlm.nih.gov/28426936/
- Mosinger J, Mosinger B. Photodynamic sensitizers assay: rapid and sensitive iodometric measurement, Experientia. 1995; 51: 106-109. PubMed: https://pubmed.ncbi.nlm.nih.gov/7875246/