More Information
Submitted: January 03, 2022 | Approved: January 25, 2022 | Published: January 26, 2022
How to cite this article: Keshavarzi A, Seifikar S, Ranjbar A, Khiripour N, Ghaleiha A, et al. Effect of fresh red grape juice and grape fermentative product on oxidative-stress in human erythrocytes in vitro. Arch Biotechnol Biomed. 2022; 6: 001-006.
DOI: 10.29328/journal.abb.1001030
Copyright License: © 2022 Keshavarzi A, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Erythrocyte; Grape; Oxidative stress; Grape fermentative product; Nutrition
Effect of fresh red grape juice and grape fermentative product on oxidative-stress in human erythrocytes in vitro
Amir Keshavarzi1, Shiva Seifikar1, Akram Ranjbar2, Nejat Khiripour3, Ali Ghaleiha1, Alireza Soltaniyan4 and Hassan Rafieemehr5*
1Research Center for Behavioral Disorders and Substances Abuse, Hamadan University of Medical Sciences, Hamadan, Iran
2Medicinal Plants and Natural Products Research Center, Hamadan University of Medical Sciences, Hamadan, Iran
3Research Center for Biochemistry and Nutrition in Metabolic Diseases, Kashan University of Medical Sciences, Kashan, Iran
4Epidemiology Department, Faculty of Health, Hamadan University of Medical Sciences, Hamadan, Iran
5Department of Medical Laboratory Sciences, School of Paramedicine, Hamadan University of Medical Sciences, Hamadan, Iran
*Address for Correspondence: Hassan Rafieemehr, Department of Medical Laboratory Sciences, School of Paramedicine, Hamadan University of Medical Sciences, Hamadan, Iran, Email: [email protected]; [email protected]
Introduction: Oxidative stress is a phenomenon induced by an imbalance between production and the biological system's ability to readily detoxify oxygen reactive species (ROS) in cells. It has been shown that grape juice can reduce oxidative stress due to the presence of polyphenols. The aim of this study was to evaluate the effect of fresh red grape juice and grape fermentative product on oxidative stress in human erythrocytes.
Methods: 5 ml of blood from 125 healthy individuals as control group collected in EDTA containing tubes. To perform biochemical assays, erythrocytes were incubated at 37 ºC for different times including 4, 24, 48, and 72 hours in the presence or absence of grape juice and grape red wine in amounts of 5 ml.
Results: Grape juice and grape red wine reduced lipid peroxidation and increase of thiol groups, and total antioxidant capacity after 24 hours of treatment (p < 0.05). Also, the activity of catalase enzyme was increased 4 and 24 hours after treatment with red wine and grape juice, respectively.
Conclusion: Grape juice and grape fermentative product may improve the antioxidant power of erythrocytes. This may lead to reducing the risk of free-radical damage and chronic diseases. However, more research with a higher number of samples is necessary to confirm the antioxidant effect of grape juice and red wine on human erythrocytes.
Today, after years of research and studies over the past decades, free radicals have been found to play a role in the pathogenesis of different diseases, including diabetes mellitus, rheumatoid arthritis, cardiovascular disease, asthma, aging, and various cancers [1]. Radicals oxygen-free (ROS) refer to molecules that have unpaired electrons in their valence layer. These molecules are highly reactive due to this electron deficiency. High levels of ROS inside cells cause damage to macromolecules including lipid, protein, and nucleic acid [2]. It has been shown that fruits and vegetables that have antioxidant properties can protect these cellular macromolecules against oxidative damage [3]. Grape is one of the most popular fruits that have been used by humans since ancient times [4]. Red wine is one of the fermented grape products. This popular global beverage is rich in antioxidants such as phenolic acids, stilbenes (trans-resveratrol), and flavonoids [5]. Previous studies have shown that there is a beneficial relationship between moderate consumption of red wine and antioxidant systems [6,7]. Polyphenols are effective antioxidant compounds found abundantly in red wine. Red-wine polyphenols have been implicated in various biological processes such as free radical elimination and inflammation modification, chemical modification, and oxidation both in the laboratory and in the natural environment [8,9]. Besides, flavonoids, procyanidin, and resveratrol in grape juice acts as an antioxidant by purifying dynamic oxygen species [4]. It has been reported that grape polyphenols have biological effects on antioxidant pathways in blood cells [10].
Erythrocytes are the most important type of blood cells that transmit oxygen from the lungs to body tissues via blood flow. Due to the absence of cell organelles particularly the nucleus and mitochondria, erythrocytes have a limited capacity of metabolism and low ability to the reducing of oxidative stress [11]. For this reason, there is critical to identify the component that improves the antioxidant capacity of erythrocytes. It seems several factors such as lifestyle and nutrition play a key role in reaching this purpose. Although the fundamental mechanisms are still poorly understood, evidence shows that grape juice has a potential to increased antioxidant capacity of erythrocytes. In this regard, Toaldo, et al. demonstrated that grape juice polyphenols can increase the antioxidants capacity of plasma and erythrocytes of humans [12]. Although the antioxidant influence of grape juice has been reported in different tissue and cells [13,14], however data on changes of oxidative stress markers in biological processes such as lipid oxidation and enzymatic reaction in healthy populations are few. In the present study, we evaluated the effects of fresh red grape juice and grape fermentation product of oxidative response in human erythrocytes by enzyme activity assessment in vitro.
Preparation of erythrocytesm
This experimental study was conducted on the erythrocyte of a healthy individual who referred to Health Center Laboratory Hamadan University of Medical Sciences. In this study, after obtaining consent to participate in the study, 5 ml of blood was collected from 125 healthy individuals (20-35 years old) as a control group in ethylenediamine tetra-acetic acid (EDTA) contained tubes. Erythrocytes were isolated by centrifugation (5 min,180 rpm at 4 °C) and washed three times with isotonic phosphate buffer (pH = 7.4). After each wash, centrifugation was performed, and the supernatant was carefully removed. Then 50 µl of erythrocytes was placed in aerobic conditions in a culture medium containing red wine and high grape juice at specified concentrations. Informed consent was obtained from each subject in this study before data collection. Control was matched by age (adults), and to have resided in the Hamadan. An inclusion criteria for controls was no previous history of any disease and residence located in Hamadan at the time of the study. The exclusion criteria for controls were including dissatisfaction with participation in the study.
Chemicals
Reagents and chemicals were provided from Sigma-Aldrich (St. Louis, MA, USA); Acetate buffer, ferric chloride, 2,4,6-tripyridyl-S-triazine (TPTZ), 2-thiobarbituric acid (TBA), tetra ethoxy propane (TEP), 5,5-Dithiobis-2-nitrobenzoic acid (DTNB), trichloroacetic (TCA), 2, 4, 6-tripyridyl- S-triazine (TPTZ), Tris base, n-butanol, hydrochloric acid (HCL), EDTA.
Analysis of erythrocyte enzymes
At intervals of 24, 48, and 72-hour medium was centrifuged, and erythrocytes were isolated.
Then they were washed three times with phosphate buffer. Erythrocytes were lysed using cold hypotonic phosphate buffer. Then, in all samples, hemoglobin concentration was adjusted to 1 g/dl. Hemoglobin concentration was measured by the cyanmethemoglobin method. To study the behavior of hemoglobin and its conformational change, the optical absorption of the samples was measured in the wavelength range of 200 to 650 nm. Oxidized forms of hemoglobin exhibit maximum optical absorption at 560, 577, and 630 nm. Furthermore, to study the interaction of heme-heme rings and heme-globin interaction, the optical absorption of the solutions was measured at 420 and 340 nm, respectively. Whole blood with anticoagulant, trisodium citrate, was prepared using cyano meth hemoglobin. Drabkin’s reagent is used to dilute blood (In the ratio of 20 µl blood to 5 ml of Drabkin).
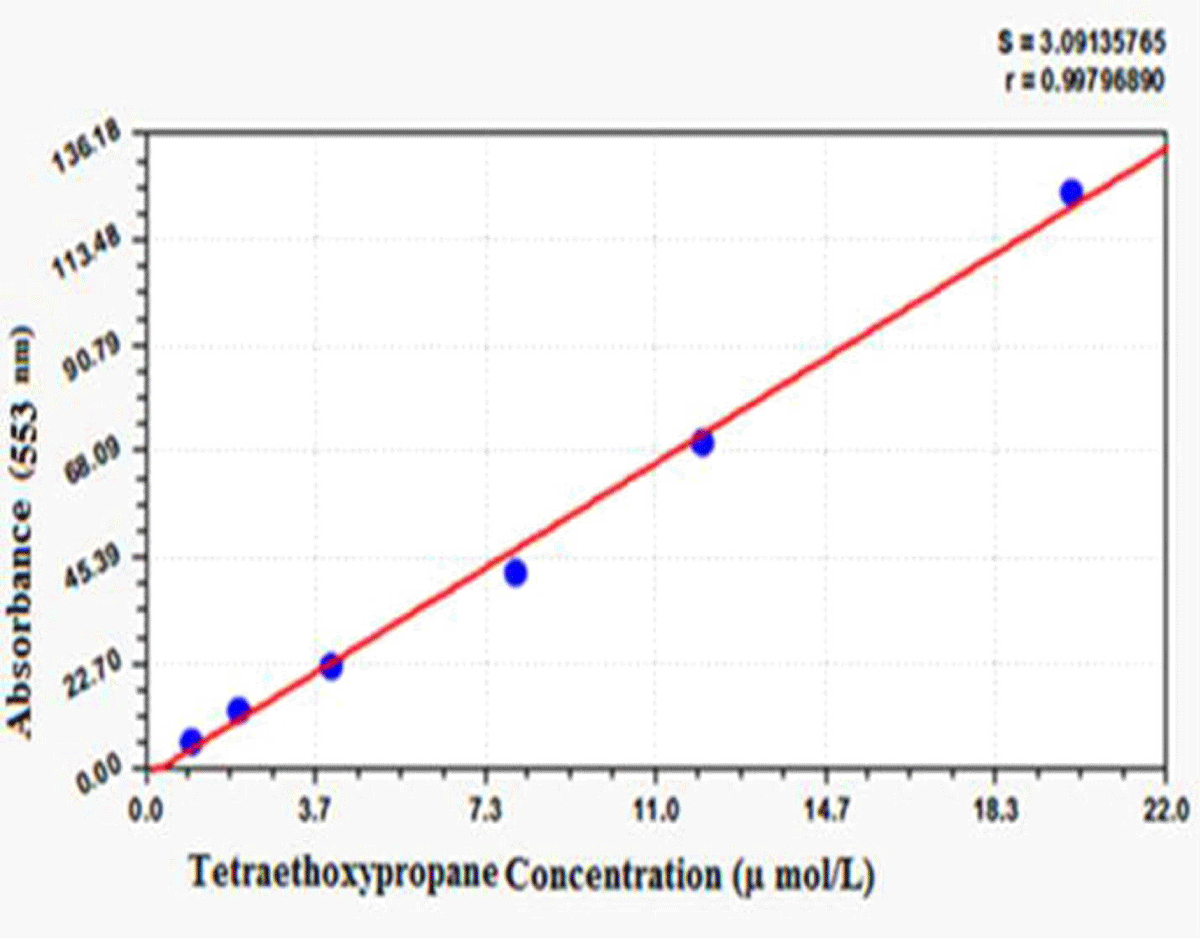
The measurements were performed with the Biovision Competitive ELISA Kit based on the kit catalog (15). Total antioxidant capacity (TAC) was measured by the ferric reducing ability of plasma (FRAP) manual method. It was determined according to the Benzie and Strain method [16]. In this method, the antioxidant compounds at low pH were measured by reduction of the Ferric Tripyridyl triazine complex (Fe III-TPTZ) to ferrous Fe II form by absorbance changes at 593 nm. In this method, 100 µg of liver tissue was added to 3 ml of FRAP reagent and read at 593 nm. The sample was then vortexed and incubated at 37 °C for 4 minutes and read again by light absorbance. All values were prepared according to Table 1. For this study, the Yagi method was performed using spectrophotometry [17]. In this method, 100 µl of the sample was added to 100 µl of SDS, 0.5 ml of acetic acid, 1.5 ml of TBA, and 200 µl of water. The tubes were then cooled with water, and 3 mL of butanol was added and shaken vigorously. The tubes containing the samples were centrifuged at 1000 g force. The supernatant containing butanol was removed. Finally, the fluorescence emission spectra were measured using fluorimetry at 553 nm. For drawing the standard curve, different dilutions of tetra ethoxy propane (TEP) were used (Supplemental Figure 1). Protein thiol (P-SH) and non-protein (GSH) groups are highly susceptible to oxidative damage. This group reduces under oxidative conditions. 2, 2-Dinitrobenzoic acid (DTNB) colorimetric method was used to evaluate the thiol groups. DNTB creates a yellow complex with these groups. This complex has maximum absorption at 412 nm. Briefly, 50 µl of homogenate was incubated with 150 µl of Tris-EDTA buffer, 10 µl of DTNB reagent and 790 µl of absolute methanol for 15 min at room temperature. It was then centrifuged at 1000 g for 10 minutes, and the supernatant absorbance was read at 412 nm (A1). To prepare the buffer, weighed 0.302 g of tris and 0.058 g of EDTA to 8 ml volume. DTNB Blanket B solution includes all of the above ingredients and steps except supernatant. Sample blank (A2) contains all first step (A1) components except DTNB. Finally, the concentration of thiol groups was calculated from the formula =1.57 mM × (A1 – A2-B).
| Table 1: Standard concentration of total antioxidant capacity test. | ||
| Standard Concentration(mM) | FeSO4 .7H2O Solution(ml) | Distilled water (ml) | 0.1 | 1 | 9 |
| 0.2 | 2 | 8 |
| 0.4 | 4 | 6 |
| 0.6 | 6 | 4 |
| 0.8 | 8 | 2 |
| 1 | 10 | 0 |
Supplemental Figure 1: Standard curve of lipid peroxidation test.
Statistical analysis
In this study, SPSS 16 software was used for data analysis. An independent t-test was used to compare observations using central indices and scatter plots and agreement tables. A paired t-test was used to compare the outcome before and after the intervention. Tests were considered significant at the 0.05 level.
Lipid peroxidation levels
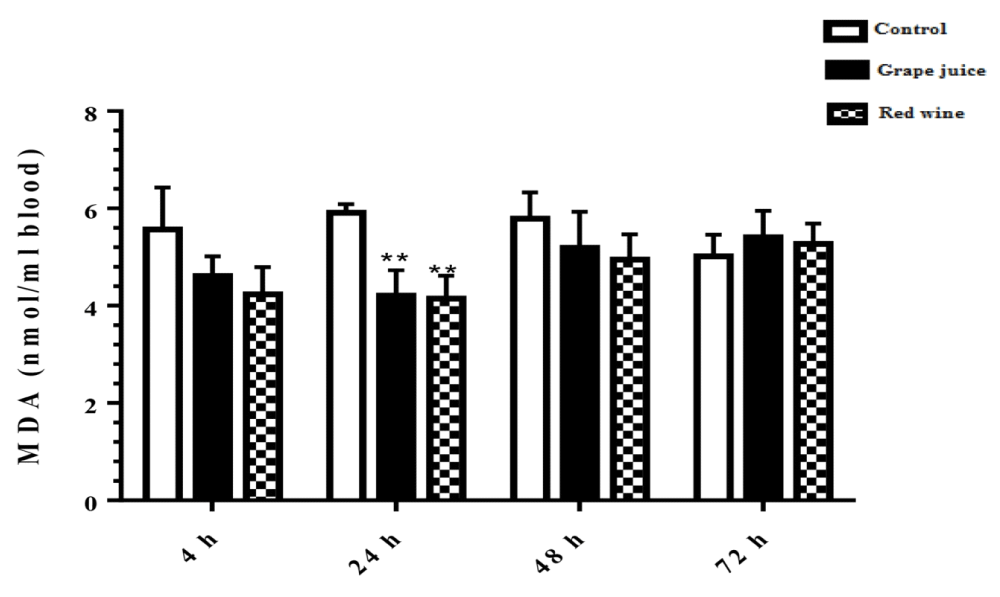
The results of lipid peroxidation in the study groups showed that in the treated groups 24 hours after the beginning of the study, the rate of lipid peroxidation under the influence of grape extract and the fermented grape product was significantly decreased compared to the control group (p < 0.01) (Figure 1).
Figure 1: Malondialdehyde (MDA) content in control and grape extract-treated groups and fermented grape products (0.15 ml/ml erythrocyte). All data are presented as SD ± Mean, *(p < 0.05), **(p < 0.01) compared to control.
Total Antioxidant Capacity (TAC)
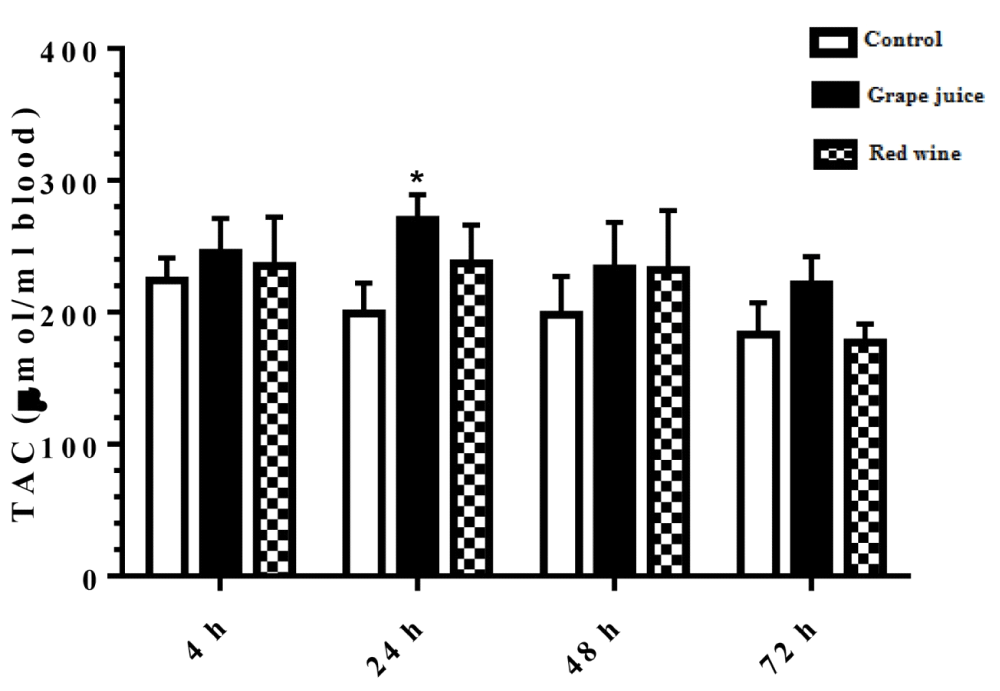
The results of the study on the effect of grape extract and fermented grape product on TAC in the study groups was shown in Figure 2. During the 24 hours after the beginning of the study, the amount of TAC was significantly increased in the groups treated with grape extract compared to the control group (p < 0.05).
Figure 2: Total antioxidant capacity (TAC) in control and treated groups with grape extract and fermented grape product (0.15 ml/ml erythrocyte). All data are presented as SD ± Mean, *(p < 0.05), **(p < 0.01) compared to control.
Total Thiol Groups (TTGs)
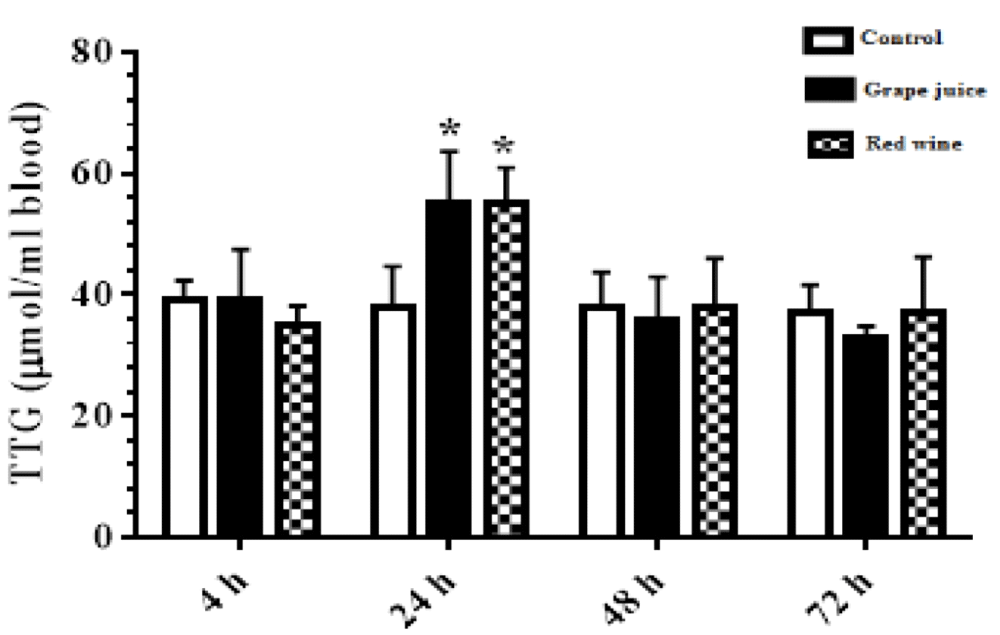
As shown in Figure 3, the results of the survey showed a significant increase in the amount of TTGs affected by grape extract and fermentation products during the 24 hours after the study (p < 0.05) (Figure 3).
Figure 3: The amount of thiol groups in control and treated groups with grape extract and fermented grape product (0.15 ml/ml erythrocyte). All data are presented as SD ± Mean. *(p < 0.05), **(p < 0.01) compared to control.
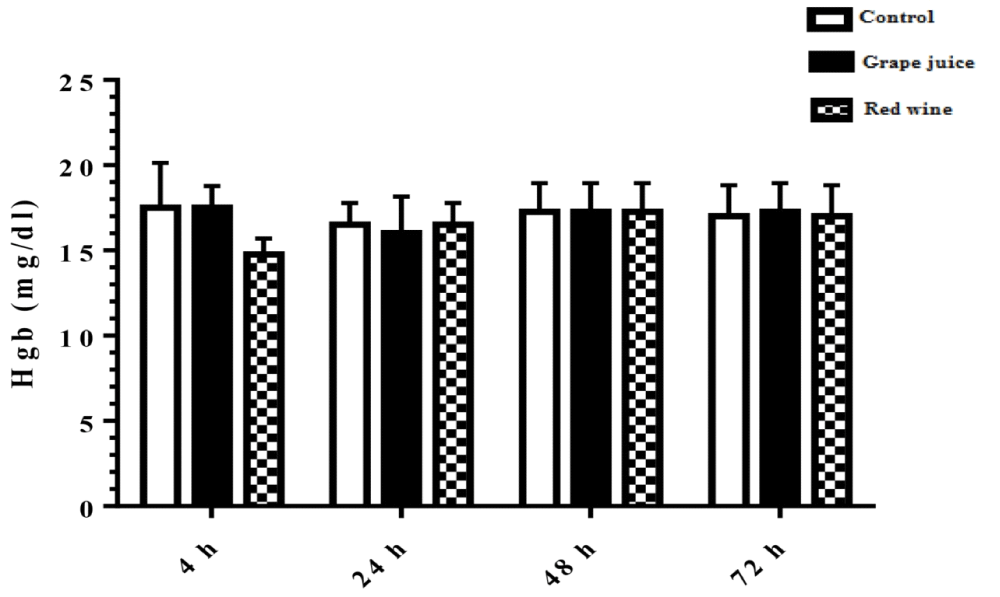
Measurement of hemoglobin levels
The results of hemoglobin levels in the study groups showed no significant difference in presence of absence of grape extract and fermentation products (Figure 4).
Figure 4: Hemoglobin level in control and treated groups with grape extract and fermented grape product (0.15 ml/ml erythrocyte). All data are presented as SD ± Mean, *(p < 0.05), **(p < 0.01) compared to control.
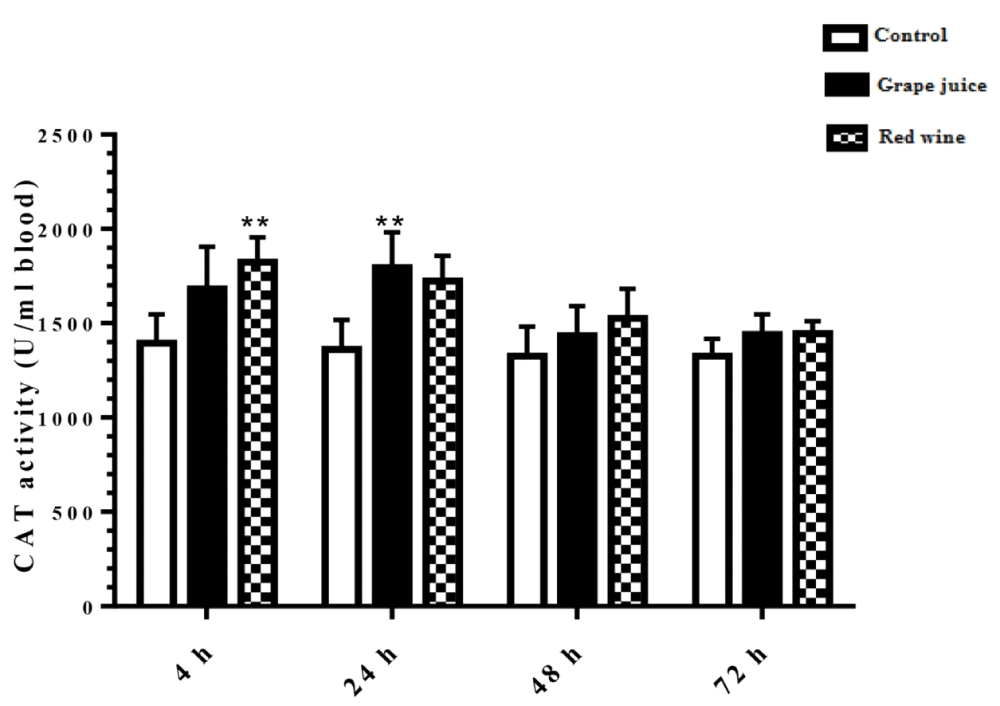
Catalase activity rate
The results of the catalase activity in the study groups showed that during the 4 and 24 hours after the beginning of the study, the fermented grape product and the grape extract, respectively, had a significant increase compared to the control group (p < 0.01) (Figure 5).
Figure 5: Catalase activity in control and treated groups with grape extract and fermented grape product (0.15 ml/ml erythrocyte). All data are presented as SD ± Mean, *(p < 0.05), **(p < 0.01) compared to control.
Oxidative stress occurs when the critical balance between ROS production and antioxidant molecules is disrupted. In this condition, cells function against the oxidant effects via activation or silencing of genes encoding defensive enzymes, transcription factors, and structural proteins [18]. It has been shown that increased oxidative stress is associated with severe cellular damage such as the accumulation of oxidation products of lipids, nucleic acids, proteins, and ultimately cellular dysfunction [19]. Membrane lipids are the first targets of various reactive oxygen. It has been reported that malondialdehyde (MDA) is one of the important lipid oxidation products of cells [20]. This oxidation product can influence gene expression and protein synthesis that these processes can lead to chronic diseases [20]. As Figure 1 shows, in our study, we found the MDA level significantly was decreased after incubation of erythrocytes with red wine. This finding is particularly important because it has been demonstrated that a high level of lipid oxidation products is involved in the development of cardiovascular diseases, cancer, neurological disorders, and also in aging [21,22]. Also, studies have shown a relationship between grape juice, which is rich in polyphenols, with a lower level of oxidative stress as well as reduction of hypertension and chronic disease incidence [15,23]. In addition, Tedesco et al. demonstrated that due to ROS attack to the erythrocyte membrane oxidation of lipids and proteins, as well as hemolysis and hemoglobin levels, increased [24]. Although our results showed a reduction of lipid peroxidation, however, we don't find any significant difference in hemoglobin level in the presence or absence of grape juice and grape red wine. In the present study, serum TAC showed an increased level 24 hours after the incubation with grape juice in comparison to the controls. As decreased TAC is reported as oxidative stress markers in plasma and erythrocytes of healthy populations during aging [25], we hypothesis that the measurement of the TAC may be the primary biochemical approach towards the evaluation of oxidative stress during lifespan. On the other hand, upon dietary intervention such as the use of vegetables and fruits, which are rich in antioxidants can improve redox homeostasis, especially in erythrocytes. The change of biological enzyme is the other important biomarker of the antioxidant status in humans. CAT, as an oxidant defense of erythrocytes, is the enzyme that converts toxic hydrogen peroxide to hydrogen and oxygen. Several studies have shown the decreased activities of these enzymes during oxidative stress as well as aging [26,27]. However, in our study CAT activity in erythrocytes was induced with grape juice and fermented grape product. This finding is consistent with studies that suggest that polyphenols in tropical juice affect antioxidant pathways including enzyme activities [28,29]. These results make grape juice a good antioxidant drink that can prevent the unfavorable effect of peroxyl radical to erythrocyte such as hemolysis or reduction of the half-life. In spite of the antioxidant activity of grape reported by previous studies as well as in Islamic Recourses [30], however, we believe that Islamic opinion of the medicinal and nutritional properties of grape juice should be considered.
Erythrocytes are constantly exposed to ROS. However, the unfavorable effect of ROS can be reduced by grape juice and fermented grape product. Several parameters are being used to evaluate the extent of the antioxidant effect of grape juice including TAC and changing of enzyme activity. Our findings indicate that the time point of 24-hour is sufficient for the antioxidant effect of grape juice and grape fermentation products. Since the change of these parameters of erythrocytes, as model cells of humans, may be an important prognostic biomarker of oxidative stress it seems a regular evaluation of these biochemical parameters be helpful in the timely prevention of oxidation-relate diseases.
Also, the fermented grape product has beneficial effects that are in line with other information in this field. One study has shown that red wine prevents the oxidative stress caused by glucose and fructose in human erythrocytes in vitro [21].
This study found that red wine reduced lipid peroxidation and increased total antioxidant power in cells. However, more research with a higher number of samples is necessary to accurately detect the antioxidant effect of grape juice and red wine on human erythrocytes.
This study was supported by a grant from Vice Chancellor of Research of Hamadan University of Medical Sciences (Grant Number: 9609075535).
Author’s contributions
H.R. has conceived the manuscript and revised it. A.K, A.S, and A.GH, N.KH provided clinical data and information. SH.S and A.R performed the technical tests, wrote the manuscript and prepared the table.
Ethical considerations
Compliance with ethical guidelines: We received the Ethical approval and observed the respective guidelines set by the Ethics Committee of Hamadan University of Medical Sciences (Ethics Code: IR.UMSHA.REC.1396.489).
- Kunwar A, Priyadarsini K. Free radicals, oxidative stress and importance of antioxidants in human health. J Med Allied Sci. 2011; 1: 53.
- Santo A, Zhu H, Li YR. Free radicals: From health to disease. React Oxyg Species. 2016; 2: 245-263.
- Nardini M, Garaguso I. Characterization of bioactive compounds and antioxidant activity of fruit beers. Food Chem. 2020; 305: 125437. PubMed: https://pubmed.ncbi.nlm.nih.gov/31499290/
- Bakota EL, Winkler-Moser JK, Berhow MA, Palmquist DE, Liu SX. Antioxidant activity of hybrid grape pomace extracts derived from midwestern grapes in bulk oil and oil-in-water emulsions. J Am Oil Chemists' Soci. 2015; 92: 1333-1348.
- Cosme F, Pinto T, Vilela A. Phenolic compounds and antioxidant activity in grape juices: A chemical and sensory view. Beverages. 2018; 4: 22.
- Berman AY, Motechin RA, Wiesenfeld MY, Holz MK. The therapeutic potential of resveratrol: a review of clinical trials. NPJ Precis Oncol. 2017; 1: 1-9. PubMed: https://pubmed.ncbi.nlm.nih.gov/28989978/
- Zuo L, Zhou T, Pannell B, Ziegler A, Best TM. Biological and physiological role of reactive oxygen species–the good, the bad and the ugly. Acta Physiol. 2015; 214: 329-348. PubMed: https://pubmed.ncbi.nlm.nih.gov/25912260/
- Arranz S, Chiva-Blanch G, Valderas-Martínez P, Medina-Remón A, Lamuela-Raventós RM, et al. Wine, beer, alcohol and polyphenols on cardiovascular disease and cancer. Nutrients. 2012; 4: 759-781. PubMed: https://pubmed.ncbi.nlm.nih.gov/22852062/
- Magrone T, Candore G, Caruso C, Jirillo E, Covelli V. Polyphenols from red wine modulate immune responsiveness: biological and clinical significance. Curr Pharma Des. 2008; 14: 2733-2748. PubMed: https://pubmed.ncbi.nlm.nih.gov/18991692/
- Magrone T, Jirillo E, Spagnoletta A, Magrone M, Russo MA, et al. Immune profile of obese people and in vitro effects of red grape polyphenols on peripheral blood mononuclear cells. Oxid Med Cell Longev. 2017; 2017: 9210862. PubMed: https://pubmed.ncbi.nlm.nih.gov/28243360/
- Arwa PS, Zeraik ML, Ximenes VF, da Fonseca LM, da Silva Bolzani V, et al. Redox-active biflavonoids from Garcinia brasiliensis as inhibitors of neutrophil oxidative burst and human erythrocyte membrane damage. J Ethnopharmacol. 2015; 174: 410-418. PubMed: https://pubmed.ncbi.nlm.nih.gov/26320685/
- Toaldo IM, Cruz FA, da Silva EL, Bordignon-Luiz MT. Acute consumption of organic and conventional tropical grape juices (Vitis labrusca L.) increases antioxidants in plasma and erythrocytes, but not glucose and uric acid levels, in healthy individuals. Nutr Res. 2016; 36: 808-817. PubMed: https://pubmed.ncbi.nlm.nih.gov/27440535/
- Balmus IM, Ciobica A, Antioch I, Dobrin R, Timofte D. Oxidative stress implications in the affective disorders: main biomarkers, animal models relevance, genetic perspectives, and antioxidant approaches. Oxid Med Cell Longev. 2016; 2016 : 3975101. PubMed: https://pubmed.ncbi.nlm.nih.gov/27563374/
- Singh CK, Siddiqui IA, El‐Abd S, Mukhtar H, Ahmad N. Combination chemoprevention with grape antioxidants. Mol Nutri Food Res. 2016; 60: 1406-1415. PubMed: https://pubmed.ncbi.nlm.nih.gov/26829056/
- Park YK, Kim JS, Kang MH. Concord grape juice supplementation reduces blood pressure in Korean hypertensive men: double‐blind, placebo controlled intervention trial. Biofactors. 2004; 22: 145-147. PubMed: https://pubmed.ncbi.nlm.nih.gov/15630270/
- Benzie IF, Szeto Y. Total antioxidant capacity of teas by the ferric reducing/antioxidant power assay. J Agric Food Chem. 1999; 47: 633-636. PubMed: https://pubmed.ncbi.nlm.nih.gov/10563944/
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979; 95: 351-358. PubMed: https://pubmed.ncbi.nlm.nih.gov/36810/
- Foyer CH. Reactive oxygen species, oxidative signaling and the regulation of photosynthesis. Environ Experimen Botany. 2018; 154: 134-42.
- Khanna P, Ong C, Bay BH, Baeg GH. Nanotoxicity: an interplay of oxidative stress, inflammation and cell death. Nanomaterials. 2015; 5: 1163-1180. PubMed: https://pubmed.ncbi.nlm.nih.gov/28347058/
- Pandey KB, Rizvi SI. Markers of oxidative stress in erythrocytes and plasma during aging in humans. Oxid Med Cell Longev. 2010; 3: 2-12. PubMed: https://pubmed.ncbi.nlm.nih.gov/20716923/
- Klinkenberg LJ, Haenen GR, Bast A, van Loon LJ, van Dieijen-Visser MP, et al. Effect of antioxidant supplementation on exercise-induced cardiac troponin release in cyclists: a randomized trial. PLoS One. 2013; 8: e79280. PubMed: https://pubmed.ncbi.nlm.nih.gov/24260184/
- Balu M, Sangeetha P, Murali G, Panneerselvam C. Age-related oxidative protein damages in central nervous system of rats: modulatory role of grape seed extract. Int J Dev Neurosci. 2005; 23: 501-507. PubMed: https://pubmed.ncbi.nlm.nih.gov/16009524/
- Yang J, Martinson TE, Liu RH. Phytochemical profiles and antioxidant activities of wine grapes. Food Chem. 2009;116: 332-339.
- Tedesco I, Russo M, Russo P, Iacomino G, Russo GL, Carraturo A, et al. Antioxidant effect of red wine polyphenols on red blood cells. J Nutr Biochem. 2000; 11: 114-119. PubMed: https://pubmed.ncbi.nlm.nih.gov/10715597/
- Carocho M, Ferreira IC, Morales P, Soković M. Antioxidants and prooxidants: effects on health and aging 2018. Hindawi. 2019.
- Kozakiewicz M, Kornatowski M, Krzywińska O, Kędziora-Kornatowska K. Changes in the blood antioxidant defense of advanced age people. Clin Interv Aging. 2019; 14: 763-771. PubMed: https://pubmed.ncbi.nlm.nih.gov/31118597/
- Maurya PK, Kumar P, Chandra P. Biomarkers of oxidative stress in erythrocytes as a function of human age. World J Methodol. 2015; 5: 216. PubMed: https://pubmed.ncbi.nlm.nih.gov/26713282/
- Cheng DM, Pogrebnyak N, Kuhn P, Poulev A, Waterman C, et al. Polyphenol-rich Rutgers Scarlet Lettuce improves glucose metabolism and liver lipid accumulation in diet-induced obese C57BL/6 mice. Nutrition. 2014; 30: S52-S8. PubMed: https://pubmed.ncbi.nlm.nih.gov/24985107/
- Petriccione M, Pagano L, Forniti R, Zampella L, Mastrobuoni F, et al. Postharvest treatment with chitosan affects the antioxidant metabolism and quality of wine grape during partial dehydration. Postharvest Bio Technol. 2018; 137: 38-45.
- Mousavi T, Rafiei A, Amjadi O, Yoosefpour M, Zakavi A. Medicinal and nutritional properties of grapes in islamic references, traditional, and modern medicine. J Mazandaran Univers Med Sci. 2015; 25: 169-190.