More Information
Submitted: October 16, 2023 | Approved: October 20, 2023 | Published: October 23, 2023
How to cite this article: Al Zubairy MA, Thamer FH, Thabet H, Aldeen KS. Production of L-Asparaginase by Yemeni Filamentous Fungi. Arch Biotechnol Biomed. 2023; 7: 018-023.
DOI: 10.29328/journal.abb.1001036
Copyright License: © 2023 Al Zubairy MA, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: L-asparaginase; Filamentous fungi; Production; Yemen
Production of L-Asparaginase by Yemeni Filamentous Fungi
Maysoon A Al Zubairy1*, Faten H Thamer2, Habib Thabet3 and Khawlah Shrf Aldeen4
1Assistant Professor of Microbiology, Microbiology Branch, Biological Sciences Department, Faculty of Science, Sana’a University, Yemen
2Assistant Professor of Chemistry, Chemistry Department, Faculty of Science, Sana’a University, Yemen
3Associate Professor of Microbiology, Ibb University, Ibb, Yemen
4Microbiology Branch, Biological Sciences Department, Faculty of Science, Sana’a University, Yemen
*Address for Correspondence: Maysoon A Al Zubairy, Assistant Professor of Microbiology, Microbiology Branch, Biological Sciences Department, Faculty of Science, Sana’a University, Yemen, Email: [email protected]
Yemen with its diverse climatic regions represents a rich resource for bioactive compounds obtained from microorganisms. This study was designed to screen fungal isolates obtained from the Microbiology branch, Biological Sciences Department, Faculty of Science, Sana’a University for their ability to produce L-asparaginase enzyme. In preliminary screening for L-asparaginase, among 77 fungal isolates about 29 fungal isolates representing 37.66% were high producers of L-asparaginase. These fungal isolates belonged to the genera Aspergillus, Eupenicillium, Fusarium, Penicillium, and Stachyobotrys. These 29 fungal isolates were screened for their ability to produce L-asparaginase using the agar well diffusion method. 12 fungal isolates out of 29 showed the ability to produce extracellular L-asparaginase. These isolates belonged to 8 species which were: A. sulphurs, A. ustus, F. sacchari, P. chrysogenum, P. citrinum, P. corylophilum, P. melinii, and P. subturcoseum. Only 5 isolates were obtained for the determination of enzymatic activity, among them P. chrysogenum showed the highest activity (279.8696U ml-1) followed by A. ustus (170.9435U ml-1). This finding is the first report on the L-asparaginase production from filamentous fungi in Yemen.
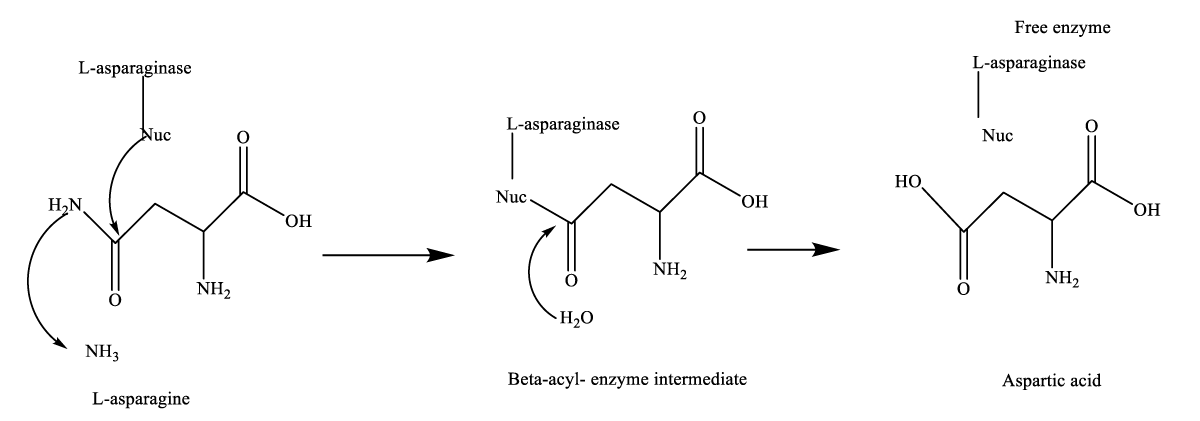
L-asparaginase (L-asparagine amido hydrolase, E.C. 3.5.1.1) is one of the amidase group enzymes that catalyses the conversion of L-asparagine to L-aspartic acid and ammonia [1]. It is known chemically as mono methoxy polyethylene glycol succinimidyl L- Asparaginase [2]. It works by deamination of the amide group located on the side chain of the non-essential amino acid, L-asparagine, leading to the formation of ammonia and L-aspartic acid [3]. This hydrolytic reaction is mostly irreversible under physiological conditions [4]. This reaction generally occurred in two steps (Figure 1). In the first step, a strong base activates the enzyme nucleophilic residue and the amide carbon atom of L-asparagine (substrate) is attacked, and a product beta-acyl-enzyme intermediate is generated. In the second step, the ester carbon is attacked by a nucleophile which is activated by a water molecule [5]. L-asparagine is a nutritional requirement of both normal and cancerous cells. Cancerous cells require high levels of this amino acid in comparison to healthy cells, due to higher division rate and metabolic processes. The low level of asparagine only affects the viability of the cancerous cells compared to the normal cells. Normal cells possess the enzyme asparagine synthetase, which is able to produce asparagine from aspartic acid, while in cancerous cells the enzyme asparagine synthetase is produced in low amounts so they are not able to produce asparagine in proper amounts. To solve this problem cancerous cells, utilize asparagine from blood [6-8].
Figure 1: General mechanism of L-asparaginase catalyzed reaction. A dashed arrow is shown by Nucleophilic attack [5].
L-asparaginase can be used in food processing which reduces the risk of acrylamide formation during frying or baking of starchy food. [9-11]. The two commercial asparaginases used in the food industry are Acrylaway produced from Aspergillus oryzae by Novozymes Company (Bagsvaerd, Denmark), and Preventase manufactured from Aspergillus niger by DSM Company (DSM Food specialties, Seclin Cédex, France). Acrylamide formation was reduced to 60% in French fries by using 10000 U.mL-1 Acrylaway when applied before frying [12]. L-asparaginases are considered one of the largest groups of therapeutic enzymes as they represent about 40% of the total worldwide sale of antileukemic and anti-lymphoma agents [13]. It is well accepted as an antitumour agent used in combination therapy with other drugs in the therapy of some lymphomas and leukemias [14-16]. It has been used for more than 30 years in the treatment of acute lymphoblastic leukemia [17]. As the enzyme is in great demand in clinical applications and in food processing industries, the demand for this therapeutic enzyme is increasing severalfold every year [18]. It is widely distributed in many plants and microbial sources, barley rootlets, and animal tissues (tissues of fishes, birds, and mammals such as liver, kidneys, pancreas, brain, spleen lungs, ovary or testes and in the serum of certain rodents) but not in man. Nevertheless, because of its wide presence in assortments of microorganisms, microbial L-asparaginase is a better source of enzyme than other living organisms, [5,19-21]. Microorganisms are the best source for obtaining enzymes as they can be cultured easily using cheap substrates, the culture conditions for enzyme production are easily optimized, and easily genetically modified to increase the yield, the enzyme can be produced in bulk, extracted, and purified economically, good stability, and consistency than animal and plant enzymes [22,23]. The toxic side effects of some currently used clinical medications of bacterial origin have necessitated the search for alternative sources of L-asparaginase [24]. Instead, eukaryotic sources such as filamentous fungi, on the other side, have revealed better compatibility with the human body, and therefore extensively explored for L-asparaginase [25]. Fungal genera of Aspergillus, Penicillium, and Fusarium have been explored as L-asparaginase producers [5,24]. Furthermore, the recombinant L-asparaginase of Aspergillus niger and A. oryzae has already been successfully utilized for the lessening of acrylamide formation in some foods [26].
Test microorganisms
Seventy-seven fungal isolates were obtained from the Microbiology Section, Biological Science Department, Faculty of Science, Sana’a University. These isolates were isolated from different soils using the dilution plate method as described by Johnson and Curel [27] collected from different governorates in Yemen and identified to genus and species level based on their macro and micro-characteristics with the help of a few works [28-32]. Confirmation of identification was carried out at the Mycological Center, Faculty of Science (MCFC), Assiut University, Egypt.
Sub-culturing and preservation of pure culture
All fungal isolates were aseptically subcultured onto Potato Dextrose Agar (PDA) plates and incubated at 28 ± 2 °C for 7 days - 10 days till the profuse fungal growth was seen. The loop full of the metabolically active culture was aseptically inoculated onto PDA slants and kept in a refrigerator at 4 ⁰C.
Preliminary screening for L-asparaginase producers
Seventy-seven fungal isolates were screened for their ability to produce asparaginase. Each fungal isolates was inoculated onto Modified Czapex Dox agar with L-asparagine as the sole source of nitrogen g/l-1: agar powder 20.0, glucose 2.0, L-asparagine 10.0, KH2PO4 1.52, KCl 0.52, MgSO4·7H2O 0.52, CuNO3·3H2O 0.001 g, ZnSO4·7H2O 0.001, FeSO4·7H2O 0.001, L-asparagine 10.0. A 2.5% stock solution of phenol red was prepared in ethanol and 3 mL of this was added to 1000 mL of Czapek Dox medium and the pH of the medium was adjusted to 6.2. Agar plates were spotted in the center with 7 days old fungal isolates. Triplicates for each isolate were prepared and Petri dishes were incubated at 28 ⁰C for 72 hrs., the appearance of a pink zone around the fungal colony in an otherwise yellow medium indicated L-asparaginase activity [33]. Control plates were maintained with medium without fungal isolates.
Production of L-asparaginase in liquid medium or secondary screening for L-asparaginase producers
Selected fungal isolates on the basis of preliminary screening were subjected to culture filtrate production in a modified Czapek Dox medium. 5 mm mycelial plug of 7-day-old culture were inoculated in 25 ml pre-sterilized MCD broth in Erlenmeyer flask under aseptic conditions and were incubated at 28 °C for 7 days - 10 days [34]. After the incubation was over, the fungal mycelium was separated from broth through filtration using Whatman filter paper No.1 followed by centrifugation at 5,000 rpm for 15 min to get cell-free culture filtrate. The cell-free culture filtrate was then used for further qualitative testing by Agar well diffusion assay.
Qualitative test by agar well diffusion assay
Agar well diffusion assay is a modified version of the Ditch Plate Assay; this technique was initially designed by Heatley [35]. Culture filtrates of selected isolates were qualitatively screened for L-asparaginase production using plate assay. L-asparaginase agar plates containing phenol red were prepared in a similar way as previously described. A six mm well was made in the center of a petri dish using a sterilized Cork borer. 30 µl of culture broth was dispensed into the well. The plates were incubated at 37 °C for 48 h. After the incubation was over, the plates were observed for the formation of a pink halo formation around the wells.
L-asparaginase production by submerged fermentation
Submerged fermentation for L-asparaginase production was carried out using modified Czapek Dox’s liquid media. Erlenmeyer flask containing 100 mL of appropriate medium was inoculated with a primary screened organism. The flasks were incubated at 30 °C at different incubation periods (24 h - 144 h). Un-inoculated media served as controls. The cultures were harvested by filtration through the Whatman No. 1 filter paper. The culture filtrate was used as a crude enzyme to estimate enzyme activity.
Estimation of L-asparaginase enzyme activity
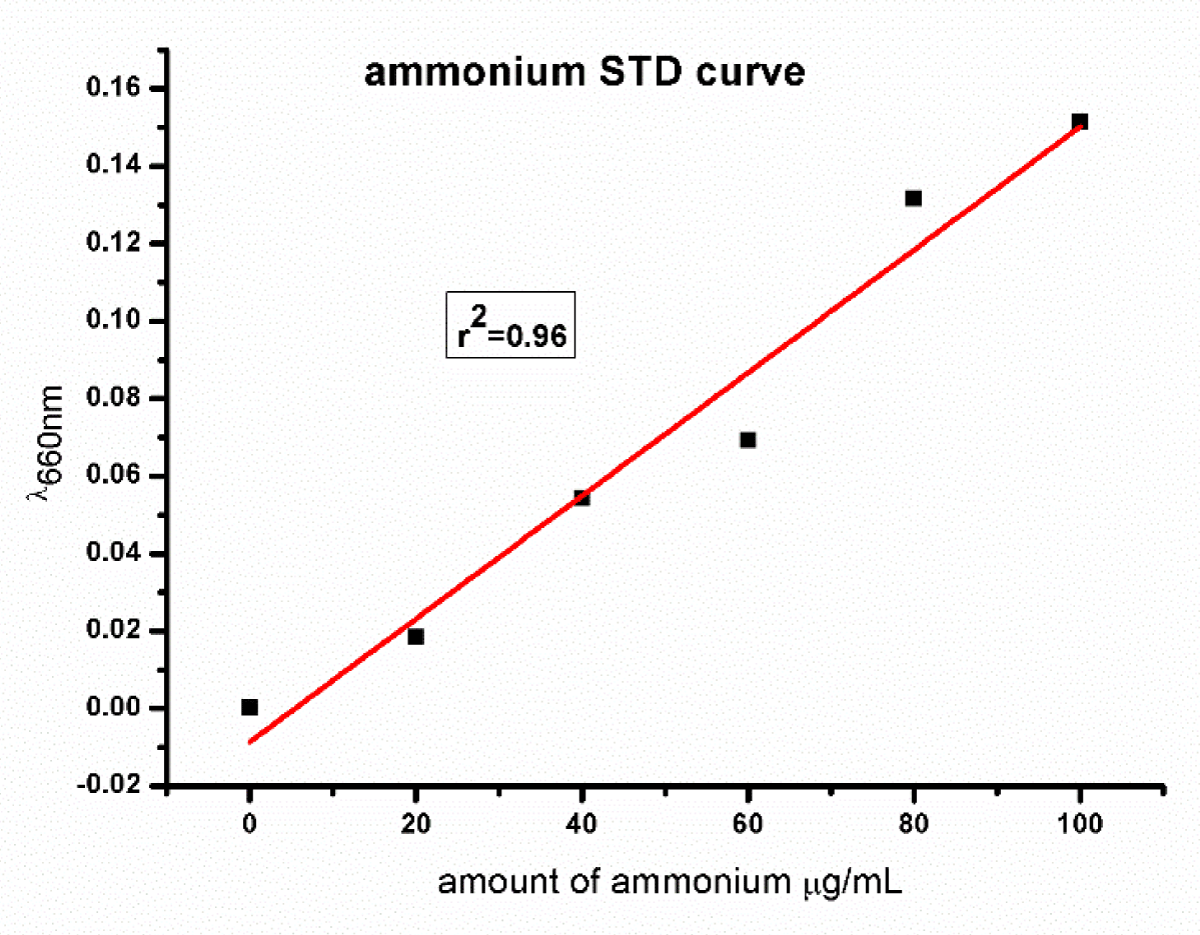
L-asparaginase activity was measured following the modified method of Imada, et al. [34]. This method utilizes the determination of ammonia liberated from L-asparagine in the enzyme reaction by Nessler’s reaction. The reaction was started by adding 0.5 ml supernatant into 0.5 ml 0.04 M L-asparagine and 0.5 ml 0.5 M phosphate buffer (pH 7), 0.5 distilled water, and 0.5 crude enzyme mixed and shacked well then incubated at 37 ⁰C for 30 min. The reaction was stopped by the addition of 0.5 ml of 1.5 M Trichloroacetic Acid (TCA). The ammonia released in the supernatant was determined calorimetrically by adding 0.2 ml Nessler’s reagent into tubes containing 0.1 ml supernatant and 3.75 ml distilled water and incubated at room temperature for 10 min, and absorbance of the supernatant was read using a UV-visible spectrophotometer (Specord200, AnalytikJena, Germany) at a wavelength of 450 nm. One unit of L-asparaginase activity is defined as the amount of enzyme that catalyzes the formation of 1 µmol of ammonia per minute under the conditions of the assay [36]. The standard curve of ammonium is shown in Figure 2.
Figure 2: Showed the ammonium standard curve.
Estimation of protein
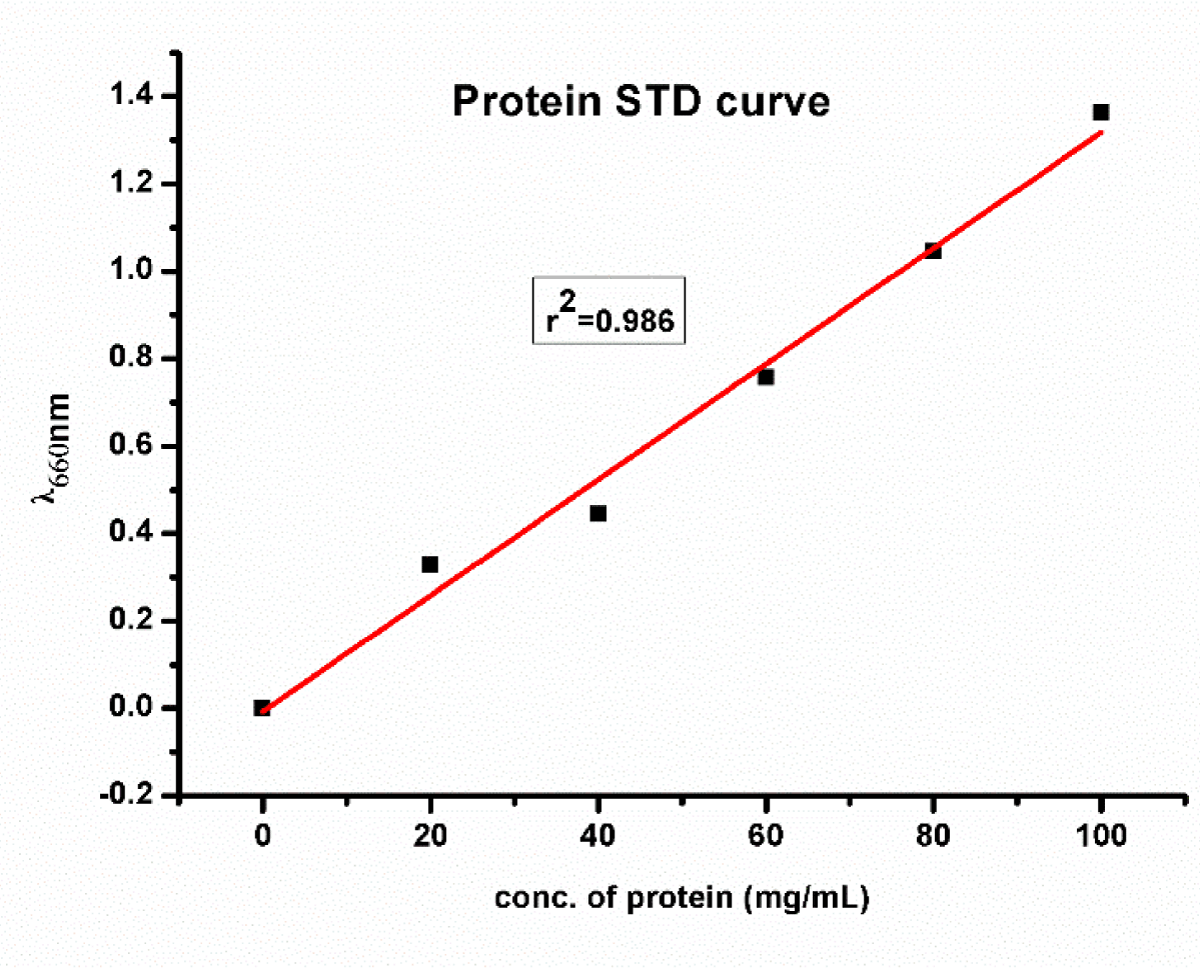
Estimation of protein was determined by using Lowry, et al. [37]. A stock solution of standard protein, BSA at a concentration of 1000 µg/mL was made. From this solution aliquots of 0.2 to 1 mL of working standard at a concentration of 100 µg/mL were taken in the test tubes. All the test tubes were made up to 1 mL with distilled water. 1 mL of FC reagents was added to each test tube. After 30 min of incubation, the absorbance was measured at 660 nm using UV-VIS spec. The standard curve of protein is shown in Figure 3.
Figure 3: Showed the Protein standard curve.
In this study, 77 fungal isolates obtained from the Microbiology Section, Biological Science Department, Faculty of Science, Sana’a University were screened for their ability to produce L-asparaginase enzyme using modified Czapex Dox agar with L-asparagine as the sole source of nitrogen and phenol red as an indicator. L- asparaginase also served as an inducer.
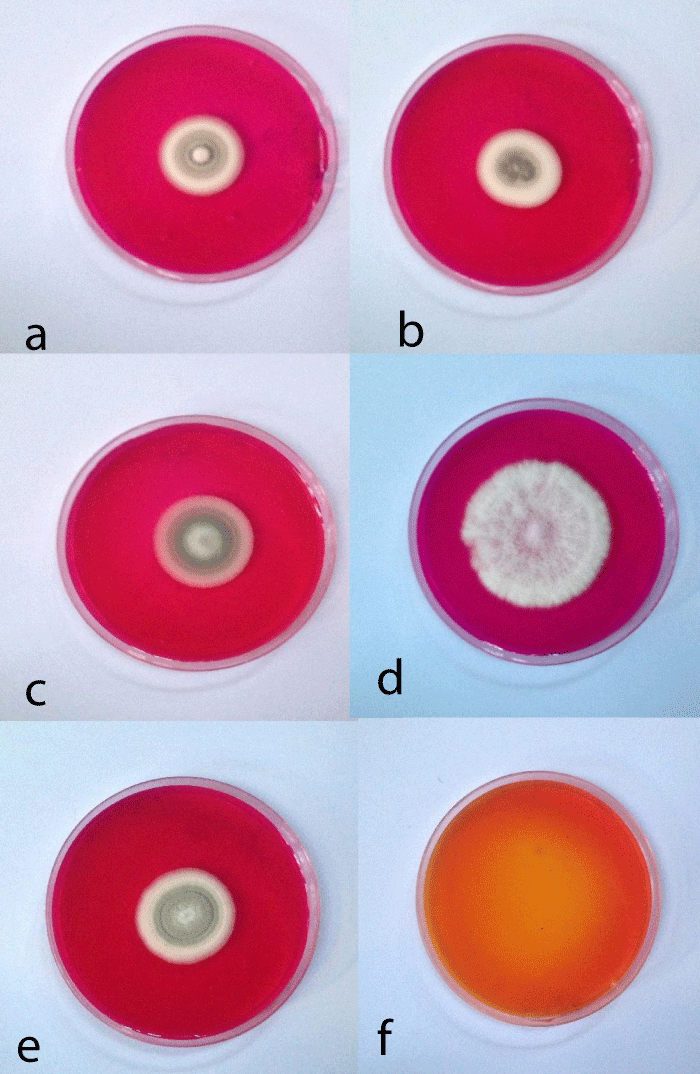
Preliminary screening of L-asparaginase production of 77 fungal isolates belonging to 27 species and 6 genera on modified Czapek’s agar medium showed that 29 fungal isolates representing 37.66% were high producers of L-asparaginase. These fungal isolates belonged to the genera Aspergillus, Eupenicillium, Fusarium, Penicillium, and Stachyobotrys. Twenty-seven fungal isolates representing 35.06% of tested isolates showed medium production of L-asparaginase, these isolates belonged to Aspergillus, Eupenicillium, Paceliomyces, and Penicillium. 7 fungal isolates (9.09%) were low producers whereas 14 fungal isolates (18.18%) showed no production of L-asperginase (Table 1). The change of medium color to red is shown in Plate 1.
| Table 1: Preliminary screening of fungal isolates for L-asparaginase production. | ||||||
| No. | Fungal isolate | Number of tested isolates | High Production | Medium Production | Low Production | No Production |
| 1 | Aspergillus alutaceous | 2 | - | - | - | 2 |
| 2 | A. aureophilum | 1 | - | 1 | - | - |
| 3 | A. melleus | 2 | - | 1 | 1 | - |
| 4 | A. nidulanus | 1 | - | - | - | 1 |
| 5 | A. sulphurs | 1 | 1 | - | - | - |
| 6 | A. terreus var. aureus | 2 | 1 | 1 | - | - |
| 7 | A. terreus var. terreus | 2 | - | - | 2 | - |
| 8 | A. ustus | 5 | 4 | 1 | - | - |
| 9 | Eupencillium shearii | 2 | - | 1 | - | 1 |
| 10 | E. sinaceum | 2 | 1 | - | - | 1 |
| 11 | Fusarium sacchari | 1 | 1 | - | - | - |
| 12 | F. semitectum | 1 | 1 | - | - | - |
| 13 | Paecilomyces lilicanus | 2 | - | 1 | - | 1 |
| 14 | Penicillium aurantiogriseum | 1 | - | 1 | - | - |
| 15 | P. chrysogenum | 11 | 5 | 6 | - | - |
| 16 | P. citrinum | 11 | 8 | 3 | - | - |
| 17 | P. corylophilum | 1 | 1 | - | - | - |
| 18 | P. expansum | 2 | - | 1 | - | 1 |
| 19 | P. funiculosum | 1 | - | - | - | 1 |
| 20 | P. glabrum | 2 | - | - | - | 2 |
| 21 | P. janthinellum | 3 | - | 1 | 2 | - |
| 22 | P. melinii Thom | 7 | 3 | 4 | - | - |
| 23 | P. oxalicum | 1 | - | 1 | - | - |
| 24 | P. spinulosum | 1 | - | - | 1 | - |
| 25 | P. subturcoseum | 2 | 2 | - | - | - |
| 26 | P. velutinum Beyma | 1 | - | - | 1 | - |
| 27 | P. verruculosum | 7 | - | 4 | - | 3 |
| 28 | Stachyobotrys elegans | 2 | 1 | - | - | 1 |
| Total count of tested isolates | 77 | 29 | 27 | 7 | 14 | |
Plate 1: Shows the preliminary screening for L-asparaginase by fungal isolates, a. P. citrinum FI 1, b. A. ustus, c. P. corylophilum,d. P. chrysogenum, e. P. citrinum FI 2 and f. medium without fungal isolates show an orange to yellow color indicating a negative result.
Twenty-nine fungal isolates that show high production of L-asparaginase were screened for their ability to produce L-asparaginase using the agar well diffusion method. 12 fungal isolates out of 29 showed the ability to produce extracellular L-asparaginase. These isolates were belonging to 8 species which they were: A. sulphurs, A. ustus, F. sacchari, P. chrysogenum, P. citrinum, P. corylophilum, P. melinii and P. subturcoseum (Table 2).
| Table 2: Secondary screening of fungal isolates for L-asparaginase. | |||
| No. | Fungal isolates | Number of tested isolates | Number of L-asparaginase producers |
| 1 | A. sulphurs | 1 | 1 |
| 2 | A. terreus var. aureus | 1 | 0 |
| 3 | A. ustus | 4 | 3 |
| 4 | E. shearii | 1 | 0 |
| 5 | E. sinaceum | 1 | 0 |
| 6 | F. sacchari | 1 | 1 |
| 7 | Pae. lilicanus | 1 | 0 |
| 8 | P. chrysogenum | 5 | 2 |
| 9 | P. citrinum | 8 | 2 |
| 10 | P. corylophilum | 1 | 1 |
| 11 | P. melinii | 3 | 1 |
| 12 | P. subturcoseum | 2 | 1 |
| Total count of tested isolates | 29 | 12 | |
5 fungal isolates that showed a positive result for secondary screening were chosen for further studies on L-asparaginase production and purification. These isolates were A. ustus, P. chrysogenum, 2 isolates of P. citrinum, and P. corylophilum, among them P. chrysogenum showed the highest activity (279.8696 U ml-1) followed by A. ustus (170.9435 U ml-1) and P. corylophilum (164.008 U ml-1) Table 3.
| Table 3: Enzymatic activity of L-asparaginase. | ||||
| Fungal isolate | Ammonium liberated µg ml-1 |
Soluble Protein content mg ml-1 |
Ammonium liberated µmol ml-1 |
L-asparaginase activity U ml-1 |
| A. ustus | 12.0666 | 80.5385 | 205.1322 | 170.9435 |
| P. chrysogenum | 19.7555 | 78.5357 | 335.8435 | 279.8696 |
| P. citrinumFI 1 | 11.2222 | 87.0571 | 190.7774 | 158.9812 |
| P. citrinumFI 2 | 4.6777 | 68.7942 | 79.5222 | 66.2685 |
| P. corylophilum | 9.9888 | 78.18 | 169.8096 | 164.008 |
Fungal isolated from soil is considered a promising source of L-asparaginase production. In this study, some fungal isolates obtained from the Microbiology Branch at the Faculty of Science, Sana’a University have been screened thoroughly for production of the L-asparaginase enzyme. Results in the present study revealed that fungal isolates P. chrysogenum showed L-asparaginase activity of 279.8696 followed by A. ustus (170.9435 U ml-1) and P. corylophilum (164.008 U ml-1) IU ml-1. These results were similar to results obtained by Bhosale and As-Suhbani [38] who reported that Penicillium sp. has an enzymatic activity of 307.114 IU ml-1 and Aspergillus sp. 216.847 IU ml-1. Our findings also agreed with Vala, et al. [39] who studied the production of L-asparaginase from a marine-derived Aspergillus niger strain AKV MKBU.
Fungi have long been known as good enzyme producers. Their absorptive mode of nutrition requires an efficient secretion of enzymes to decompose nutrients in the extracellular medium so that the presence of an inducer substrate usually activates the production of the enzyme required for its utilization. This makes fungi potential sources for the production of extracellular enzymes [40]. It has been observed that eukaryotic microorganisms like yeast and fungi have a potential for asparaginase production [41,42] For example, the mitosporic fungi genera such as Aspergillus, Penicillium, and Fusarium, are commonly reported in scientific literature to produce asparaginase [43,44].
Yemen with its diverse climatic region represents an excellent resource for bioactive compounds derived from microorganisms, but unfortunately, there are only a few available studies about this subject [45,46]. This is because of the circumstances of the country as a result of conflict and the lack of resources for studying these bioactive compounds which make it a difficult issue for Yemeni researchers because they need more effort than other fields of Microbiology. More studies must be done in the future for the characterization of L-asparaginase and the production of it from alternative resources such as waste.
L-Asparaginase represents a good antitumor agent with effective treatment of lymphosarcoma and lymphoblastic leukemia. This enzyme could be obtained from different species which represent a good source for L-Asparaginase. Tested fungal isolates showed the ability to produce this enzyme either on solid or liquid media. Fungal isolates showed a high enzymatic activity when studied in submerged medium.
- Ghasemi Y, Ebrahimminezhad A, Amini SR, Zarrini G, Ghoshoon MB, Raee MJ, Morowvat MH, Kafilzadeh F, Kazemi A. An optimized medium for screening of L-asparaginase production by Escherichia coli. Amer J Biochem Biotechnol. 2008; vol: 4(4): 422-24.
- Niharika Y, Supriya S. Production of L-Asparaginase by Fusarium Oxysporum Using Submerged Fermentation. International Journal of Pharmaceutical Science Invention. 2014; 3(6): 32-40.
- Zuo S, Zhang T, Jiang B, Mu W. Recent research progress on microbial L-asparaginases. Appl Microbiol Biotechnol. 2015 Feb;99(3):1069-79. doi: 10.1007/s00253-014-6271-9. Epub 2014 Dec 11. PMID: 25492420.
- Siddalingeshwara KG, Lingappa K. Production and characterization of L-Asparginase a tumor inhibitor.Int. J. Pharm Tech Res. 2011; 3: 314-319.
- Cachumba JJ, Antunes FA, Peres GF, Brumano LP, Santos JC, Da Silva SS. Current applications and different approaches for microbial l-asparaginase production. Braz J Microbiol. 2016 Dec;47 Suppl 1(Suppl 1):77-85. doi: 10.1016/j.bjm.2016.10.004. Epub 2016 Oct 27. PMID: 27866936; PMCID: PMC5156506.
- Sahu MK, Poorani E, Sivakumar K, Thangaradjou T, Kannan L. Partial purification and anti-leukemic activity of L-asparaginase enzyme of the actinomycete strain LA-29 isolated from the estuarine fish, Mugil cephalus (Linn.). J Environ Biol. 2007 Jul;28(3):645-50. PMID: 18380089.
- Dharmsthiti SC, Luechai S. Purification and characterization of asparaginase from solidstate culture of Aspergillus Niger AK10. International Journal of Biotechnology and Biochemistry. 2010; 6(7):1083-1092.
- Theantana T, Hyde KD. Lumyong S. Asparaginase production by endophytic fungi from Thai medicinal plants: cytotoxic properties. International Journal of Integrative Biology.2009; 7(1): 1-8.
- Kumar Jha S, Divya P, Rati KS, Hare RS, Vinod KN, Ambrish SV. Microbial l-asparaginase: ‘Review on current scenario and future prospects. International Journal of Pharmaceutical Science and Research. 2012; 3(9): 3076-3090.
- Trilokchandran B., Agrawal P, Krishna V. Screening studies of microbial species for L-asparaginase production. J. Environ. Res. Dev. 2016; 10(4): 712-716.
- Xu F, Oruna-Concha MJ, Elmore JS. The use of asparaginase to reduce acrylamide levels in cooked food. Food Chem. 2016 Nov 1; 210:163-71. doi: 10.1016/j.foodchem.2016.04.105. Epub 2016 Apr 22. PMID: 27211635.
- Pedreschi F, Kaack K, Granby K. The effect of asparaginase on acrylamide formation in French fries. Food Chem. 2008 Jul 15;109(2):386-92. doi: 10.1016/j.foodchem.2007.12.057. Epub 2007 Dec 28. PMID: 26003362.
- Warangkar SC, Khobragada CN, Dawane BS, Bhosale RB. Effect of dihydropyrimidine dervatives on kinetic parameters of E. carotovora L-asparginae. Int J Biotechnol. App. 2009; 1:5-13.
- Jha SK, Pasrija D, Sinha RK, Singh HR, Nigam VK, Vidyarthi AS. Microbial L-Asparaginase: A Review On Current Scenario and Future Prospects. International Journal of Pharmaceutical Sciences and Research. 2012; 3: 3076-3090.
- Arjun JK, Aneesh BP, Harikrishnan K. Sequencing and characterization of L-asparaginase (ansB) gene of Bacillus megaterium isolated from Western Ghats, Kerala, India. International Journal of Current Microbiology and Applied Sciences. 2015; 4: 753-760.
- El-Naggar Nel-A, Moawad H, El-Shweihy NM, El-Ewasy SM. Optimization of Culture Conditions for Production of the Anti-Leukemic Glutaminase Free L-Asparaginase by Newly Isolated Streptomyces olivaceus NEAE-119 Using Response Surface Methodology. Biomed Res Int. 2015; 2015:627031. doi: 10.1155/2015/627031. Epub 2015 Jun 9. PMID: 26180806; PMCID: PMC4477217.
- Ghasemi A, Asad S, Kabiri M, Dabirmanesh B. Cloning and characterization of Halomonas elongata L-asparaginase, a promising chemotherapeutic agent. Appl Microbiol Biotechnol. 2017 Oct;101(19):7227-7238. doi: 10.1007/s00253-017-8456-5. Epub 2017 Aug 11. PMID: 28801829.
- Gurunathan B, Sahadaevan R. Optimization of media components and operating conditions for exogenous production of fungal L-asparaginase. Chiang Mai J Sci 2011; 38:270-279.
- Adamson RH, Fabro S. Antitumor activity and other biologic properties of L-asparaginase (NSC-109229)-a review. Cancer Chemother Rep. 1968 Oct;52(6):617-26. PMID: 4895425.
- Cooney DA, Handschumacher RE. L-asparaginase and L-asparagine metabolism. Annu Rev Pharmacol. 1970; 10:421-40. doi: 10.1146/annurev.pa.10.040170.002225. PMID: 4911021.
- Emmanuel E, Nzelibe HC, Onyike E. Isolation, Partial Purification and Characterization of L-Asparaginase from Hedgehog Serum. Journal of Microbial & Biochemical Technology. 2015; 7: 404-409.
- Savitri N, Asthana N, Azmi W. Microbial L-asparaginase a potent antitumor enzyme. Ind. J. Biotechnol. 2003; 2:184-194.
- Verma N, Kumar K, Kaur G, Anand S. L-asparaginase: a promising chemotherapeutic agent. Crit Rev Biotechnol. 2007 Jan-Mar;27(1):45-62. doi: 10.1080/07388550601173926. PMID: 17364689.
- Mishra A. Production of L-asparaginase, an anticancer agent, from Aspergillus niger using agricultural waste in solid state fermentation. Appl Biochem Biotechnol. 2006 Oct;135(1):33-42. doi: 10.1385/abab:135:1:33. PMID: 17057254.
- Dange V, Peshwe S. Purification and biochemical characterization of L-asparaginase from Aspergillus niger and evaluation of its antineoplastic activity. International Journal of Science and Research. 2015; 564–569.
- Pedreschi F, Mariotti S, Granby K, Risum J. Acrylamide reduction in potato chips by using commercial asparaginase in combination with conventional blanching. LWT—Food Science and Technology. 2011; 44:1473–1476. DOI 10.1016/j.lwt.2011.02.004.
- Johnson LF, Curel EA. Methodes for research on ecology of soil-borne pathogens. Burgess Publishing Co., Minnepolis, M N, USA. 1973.
- Raper KB, Fennell DI. The genus Aspergillus. Krieger Publishing Company. Huntingon, New York, USA. 1977.
- Pitt JI. The genus Penicillium and its telemorphic states Eupenicillium and Talaramyces. Academic Press. London. 1979.
- Moubasher AH. Soil fungi in Qatar and other Arab countries. The Centre of Scientific and Applied Research. University of Qatar. Doha, Qatar. 1993.
- Samson RA, Hoekstra ES, Frisvad JS. and Filtenborg, O. Introduction to food-borne fungi. Centraalbureau voor schimmelcultures Baarn Delft. 1995.
- Watanabe T. Pictorial atlas of soil and seed fungi. Morphological of cultured fungi and key to species. CRC Press LLC. USA. 2002.
- Gulati R, Saxena RK, Gupta R. A rapid plate assay for screening L-asparaginase producing micro-organisms. Lett Appl Microbiol. 1997 Jan;24(1):23-6. doi: 10.1046/j.1472-765x.1997.00331x. PMID: 9024001.
- Imada A, Igarasi S, Nakahama K, Isono M. Asparaginase and glutaminase activities of micro-organisms. J Gen Microbiol. 1973 May;76(1):85-99. doi: 10.1099/00221287-76-1-85. PMID: 4723072.
- Heatley NG. A method for the assay of penicillin. Biochem J. 1944;38(1):61-5. doi: 10.1042/bj0380061. PMID: 16747749; PMCID: PMC1258024.
- Theantana TKD, Hyde S, Lumyong KMITL. Sci. Tech. J‖. 2007; 7: 13-18.
- LOWRY OH, ROSEBROUGH NJ, FARR AL, RANDALL RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265-75. PMID: 14907713.
- Bhosale H, As-Suhbani A. Screening of fungal endophytes isolated from medicinal plants for glutaminase free L-asparaginase activity. Journal of Experimental Biology and Agricultural Sciences. 2019; 7(4): 396-402.
- Vala A, Dudhagara D, Dave B. Enhanced L-asparaginase production by a marine-derived eurihaline Aspergillus niger strain AKV MKBU- a statical model. Indian Journal of Geo Marine Science. 2016; 47(06):1172-1179.
- Bacelar A, Maia A, Rueda J, Conte Vanzela A. Fungal production of the anti-leukemic enzyme L-asparaginase: from screening to medium development, Maringá. 2016; 38(4):387-394.
- Wade HE, Robinson HK, Phillips BW. Asparaginase and glutaminase activities of bacteria. J Gen Microbiol. 1971 Dec;69(3):299-312. doi: 10.1099/00221287-69-3-299. PMID: 5004160.
- Pinheiro IO, Araujo JM, Ximenes ECPA, Pinto JCS, Alves TLM. Production of L-asparaginase by Zymomonas mobilis strain CP4. Biomaterial and Diagnostic BD06. 2001; 243-244.
- Carta de-Angeli L, Pocchiari F, Russi S, Tonolo A, Zurita VE, Ciaranfi E, Perin A. Effect of L-asparaginase from Aspergillus terreus on ascites sarcoma in the rat. Nature. 1970 Feb 7;225(5232):549-50. doi: 10.1038/225549a0. PMID: 5411861.
- Arima K. Igarasi S, Nakahama K, Isonom M. Production of extracellular L-asparaginases from microorganisms. Agricul. Biol.Chem. 1972; 36:356-361.
- Al-Zubairy MA, Hussein K, Alkhyat SH, Al-Mahdi AY, Alghalibi SM, Al-Gheethi AA, Al-Shaibani MM, El Enshasy HA, Sidik NM. Antibacterial Activity of a Novel Oligosaccharide from Streptomyces californics against Erwinia carotovora subsp. Carotovora. Molecules. 2022 Apr 7;27(8):2384. doi: 10.3390/molecules27082384. PMID: 35458585; PMCID: PMC9032947.
- Yusuf Q, Al-Maqtari M, Al-Mahdi A, Al-Awadhi O. New Records of Streptomyces and Non Streptomyces Actinomycetes Isolated from Soils Surrounding Sana'a High Mountain. International Journal of Research in Pharmacy and Biosciences. 2016; 3: (3): 19-3.