More Information
Submitted: July 24, 2023 | Approved: June 17, 2024 | Published: June 18, 2024
How to cite this article: Dash N, Bajhaiya AK, Chandrashaker B, Gugulothu P. Microalgal Derivatives as Potential Nutraceutical and Pharmaceutical: Boon to Human Beings. Arch Biotechnol Biomed. 2024; 8: 017-026.
DOI: 10.29328/journal.abb.1001040
Copyright License: © 2024 Dash N, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Microalgae; Carbohydrates; Proteins; Lipids; Omega fatty acids
Microalgal Derivatives as Potential Nutraceutical and Pharmaceutical: Boon to Human Beings
Namrata Dash1, Amit Kumar Bajhaiya2, Chandrashaker B3 and Poornachandar Gugulothu1*
1Department of Biotechnology, Central University of Tamil Nadu, Thiruvarur, 610005, Tamil Nadu, India
2Department of Microbiology, Central University of Tamil Nadu, Thiruvarur, India
3Department of Microbiology, Osmania University, Hyderabad – 500007, Telangana, India
*Address for Correspondence: Poornachandar Gugulothu, Department of Biotechnology, Central University of Tamil Nadu, Thiruvarur, 610005, Tamil Nadu, India, Email: [email protected]
Background: Marine resources have diverse biological and beneficial entities for human beings. Among them, microalgae are one of the eukaryotic photosynthetic organisms found in freshwater and marine environments with an immense source of metabolites. They consist of high nutraceutical and value-added compounds for health concerns.
Objective: Most microalgal species like- Chlorella, spirulina, Isochrysis, Nannochloropsis, etc. are found to synthesize biologically active secondary metabolites which are having high pharmaceutical and nutraceutical value. Some of the purely extracted compounds are Lecithin, fucoxanthin, astaxanthin, and most important Sulphur polysaccharides- fucose, galactose, xylose, rhamnose, etc. are providing anti-microbial, anti-fungal, anti-viral, anti-cancer and anti-diabetic activities.
Methods: Many of the prior studies demonstrated the compilation of naturally derived secondary metabolites for their potential application in most fields. Because of their wide-ranging potential for use in biopharmaceutical and nutraceutical industries, microalgae have recently gained significant interest on a global scale.
Result: Microalgae are both parts of the dietary ingredients and bioactive pharmaceuticals. A number of microalgal species have been explored for their significance towards their high-value products with their exceptional pharmacological and biological properties.
Conclusion: This current review discussed the uses and benefits of microalgae for the manufacture of nutraceuticals and the medicinal use of diverse bioactive compounds.
A multiphyletic cluster of complex photosynthetic beings performs a major role in the biogeochemical cycle and complex food web referred to as algae. These are among the most molecularly diverse groups of organisms on this earth as they comprise a variety of macroscopic and microscopic organisms that are distributed across the tree of life involving prokaryotic and eukaryotic forms [1]. Each major group of algae is diagnosed by its pigments [2]. Bluegreen algae consist of a lot of pigments i.e., phycobiliproteins, allophycocyanin, phycocyanin, chlorophyll-a, -d, -f, and also phycoerythrin. Organisms belonging to Chlorophyta contain similar chlorophyll-a & b, carotenoids like β-carotene, and different xanthophylls-astaxanthin, zeaxanthin, lutein, and canthaxanthin, etc. The 3rd class Rhodophytes as red algae producing a large spectrum of light-harvesting pigments carotenes and xanthophyll. Still, they are devoid of any accessory chlorophylls. The actual reason is the primary pigments found are phycoerythrin, and phycocyanin which are able to mask chlorophyll-a. Rather than cyanobacteria are identified with greater than 200 bioactive metabolites [3], and thousands more belongs to eukaryotic microalgae. These metabolites are providing broad range of biotechnological applications involving biodiesel manufacture, wastewater bioremediation, diet related supplements for nutrition of animal and human beings [4]. Current economic viability brought about two major drawbacks i.e., the production of limited biomass leads to higher expenditure commercially. This can only be cost-effective by the process of biofuel production synergistic to higher value co-products commercialization. Comparatively, it is a rapidly growing market as the need for microalgae biomass for nutraceutical, and pharmaceutical product formulations gradually increase. In the last few decades, a lot of effort & money has been spent on screening and characterization of microalgal bioactive metabolites [5]. That results in the discovery and purification of a huge number of microalgae-derived bioactive metabolites such as sulfated polysaccharide, marennine, and different carotenoids like fucoxanthin, astaxanthin, β-carotene, polyphenols, omega-3 fatty acids, etc. from the marine ecosystem [6]. These compounds perform immense biological activities including anti-inflammation, antiviral, anti-cancerous, and antioxidant characteristics [7]. Hence, instead of synthetic dietary supplements naturally derived metabolites from microalgae also carry out the prevention, management & correction of physiological aberrations and act as a sustainable source of human diet supplements [8]. However massive cultivation of microalgae and biorefinery methods led to the evolution of economic and biological challenges that need to be addressed to enable the sustainable development of many valuable products with health and nutritional advantages.
The economic value of microalgae and their metabolites has been increasingly recognized due to their applications in biofuels, nutraceuticals, pharmaceuticals, and other industries. The global market for microalgae is projected to reach significant growth, with estimates suggesting a value of around $1.1 billion by 2028. Microalgae-derived products, such as biofuels, can be economically viable when crude petroleum prices exceed $100 per barrel. For biofuels, the production cost is competitive, with algal bioethanol costing about $2.95 per gallon and biodiesel around $5 per gallon gasoline equivalent. High-value metabolites from microalgae, including carotenoids, antioxidants, and fatty acids, enhance the nutritional value of human and animal diets. The production costs for these metabolites can vary, but efficient biorefining and valorization methods are essential for maximizing their economic potential [9](Khan, 2018).
Microalgae are a rich source of various bioactive metabolites, each with specific biological activities. The composition of microalgae includes:
1. Proteins (15% - 60%): Essential amino acids for nutrition.
2. Carbohydrates (10% - 50%): Energy source and biofuel production.
3. Lipids (1% - 40%): Including omega-3 and omega-6 fatty acids.
Bioactive metabolites include:
- Carotenoids (0.1% - 1%): Antioxidant, antidiabetic.
- Phycobiliproteins (0.5% - 5%): Antioxidant, anticancer.
- Polyunsaturated fatty acids (PUFAs, 1% - 5%): Anti-inflammatory, cardiovascular health.
- Phenolics and flavonoids (0.1% - 5%): Antimicrobial, wound healing, antineurodegenerative.
The focus on specific compounds like carotenoids, PUFAs, and phenolics is due to their potent biological activities and significant therapeutic potential across various applications such as antimicrobial, antidiabetic, wound healing, and antineurodegenerative treatments [9](Khan, 2018).
Microalgae, microscopic algae rich in essential nutrients, are gaining recognition as a superfood. They are celebrated for their high content of proteins, vitamins, minerals, and essential fatty acids. Among the various types of microalgae, Arthrospira (commonly known as spirulina) and Chlorella stand out in the food industry for their extensive use and well-documented health benefits. spirulina and Chlorella are commonly available in supplement forms, such as tablets, capsules, and powders. These supplements are popular for their concentrated nutritional benefits and are used by those looking to improve their overall health and wellness. Nutritional Content: Both spirulina and Chlorella are packed with nutrients. They contain high levels of protein, essential amino acids, vitamins (such as B12), minerals (like iron and magnesium), and antioxidants. This makes them particularly attractive for enhancing the nutritional content of various food products. The prominence of spirulina and Chlorella in the food industry is attributed to their exceptional nutritional profiles, sustainable cultivation methods, established safety, and numerous health benefits. As research continues, the food industry may begin to explore other microalgae species, potentially expanding the range of algae-based superfoods available to consumers [10].
Biomass complexity
A major portion of aquatic and freshwater phytoplankton are occupied by microalgae; the photosynthetic eukaryote occupies primary trophic strata. Many marine life feed upon them and fulfill their nutrition requirement. Microalgal biomass comprises various lipids, pigments, ω-3 fatty acids, carotenoids, and other fine chemicals [11]. Their wide evolutionary history and diversified adaptation in a broad range of habitats and survival in extreme ranges from deep hydrothermal vents to cold led scientists to use them for drug discovery. The reason behind the production of unique compounds from microalgae lies in response to communication, defense, and survival that may not have any terrestrial equivalents [12]. Over 100,000 species of Diatoms from the Microalgae group are identified in virtually every water consisting environment not just oceans and lakes but also in soil. There are numerous proven applications for diatoms: such as in biofuels, biomolecules, nanotechnology-derived materials, healthy foods, and biological water remediation techniques [13]. However, there is very little information reported about diatoms pharmaceutical applications. Different studies suggested that certain diatoms are abundant in bioactive metabolites likewise a sulfated polysaccharide called naviculan extracted from Navicula directa possesses antiviral action [14], adenosine acts as an antiarrhythmic substance isolated from Phaeodactylum tricornutum against tachycardia. Haslea ostrearia, a marine diatom synthesizes a blue colour pigment named marennine possesses a number of pharmacological properties e.g., antibacterial, antioxidant, allelopathic, antiviral, and growth-inhibiting properties [15]. It is also observed that NAMO- nonyl 8-acetoxy-6-methyloctanoate, a fatty alcohol ester isolated from diatom and marine carotenoid fucoxanthin from brown seaweeds performs anticancer, antiangiogenic, antidiabetic, antioxidant, anti-obesity, anti-inflammatory and antimalarial properties [7].
Microalgae extracted Edible compound
Growing acceptance of whole-biomass products, commercially marketed as ‘superfoods’ is ascribed to claims about their protein-rich quantity, and nutritional quality as a boon to the health of human beings. One such example is Arthrospira sold in the market as a good resource of phycocyanin, ϒ-linolenic acid, and higher-quality protein. Additionally, Arthrospira is also renowned for its antioxidant, anticancer, antiviral, and anti-inflammatory action among other benefits [16]. Besides the advantageous properties, numerous studies also suggested that consumption of excessive doses of Arthrospira or Chlorella put forth a negative impact on individuals. It was discovered that Chlorella causes acute tubulointerstitial nephritis and leads to kidney failure [17]. Similarly, excessive intake of Arthrospira poses adverse side effects including nausea, vomiting, and diarrhea. Therefore numerous questions arise regarding the safety intake of other microalgal strains for human beings because of possibly harmful side effects raised by above mentioned cases [18]. Moreover, a number of bioactive compounds are still to be investigated. There is urgent need to identify potentially toxic metabolites and to develop new strategies for extraction of targeted compounds from microalgae rather than using entire biomass.
Biochemistry of microalgae
An array of molecular compounds including lipids, carbohydrates, nucleic acids, and proteins; besides necessary vitamins and minerals are also synthesized by microalgae. Depending on the type of strain of algae, abiotic and biotic components affecting the environment e.g., photoperiod, temperature, light intensity, nutrients, and growth phase they are exposed to, the composition of each cellular fraction changes.
Proteins
Proteins perform a major role in structural buildup and metabolism in microalgal cells. On the basis of the quantity of dry biomass, several species of microalgae are analyzed to produce bulk concentrations of proteins. This concentration may vary from 42% to more than 70% in the case of specific Blue-green algae, around 58% for Chlorella vulgaris [19]. Compared to quantity, quality is more well-balanced. They are able to develop all amino acids that are essential to mammals. Furthermore, the amino acid index is similar to the lactoglobulin, soy, and egg albumin-like high-quality protein sources.
Carbohydrates
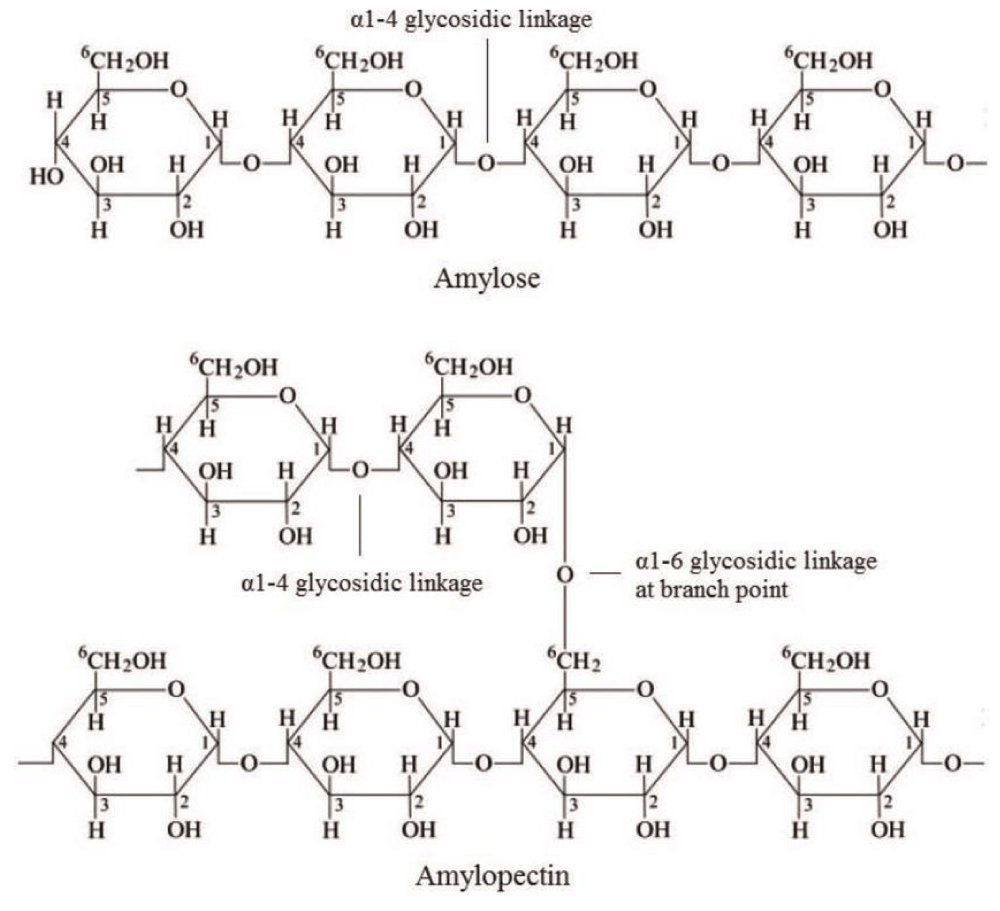
Mono-, oligo- and polysaccharides are the building blocks of Carbohydrates that perform an important role in the structural organization and metabolic regulation of organisms (Figure 1). Microalgae carry out photosynthesis to produce carbohydrates and store energy in the form of starch and glucose. Despite the fact some species produce semi-amylopectin, cyanophytes are also known to store Glycogen [20]. Microalgae belonging to Chlorophyta produce starch in the form of two glucose polymers i.e., amylopectin and amylose [21]. Floridean starch is a type of carbohydrate polymer synthesized by Rhodophyta [22]. Heterokontophyta and Bacillariophycae belong to diatoms manufacture a long chain polymer of glucose units having β (1,3) & β (1,6) linkage, Chrysolaminarin. Although all of these represent a beneficial reservoir of carbohydrates, still their application in the food industry is much less extended.
Figure 1: Carbohydrates.
Lipids
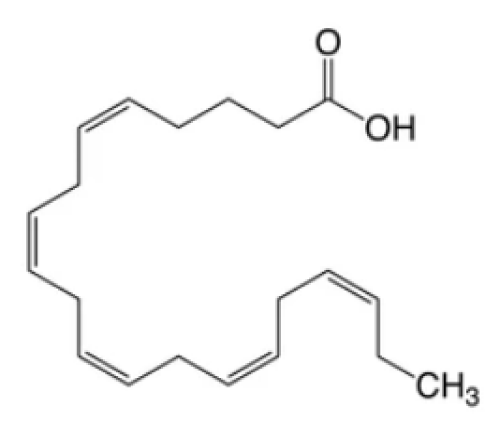
From the perspective of extraction and commercialization, lipids have drawn strong interest among different chemical elements. The commercial worth of polyunsaturated fatty acids, like ω-fatty acids (Figure 2) is significantly higher in infant formulations as well as in nutraceuticals [23]. Lipids constitute the base of the plasma membrane structure. Focusing on lipid components of Microalgae, they are mainly made of two types i.e., 1. Neutral lipids- Unbound fatty acids, acylglycerols, β-carotene (carotenoids) 2. Polar lipids- different Phospholipids and galactolipids. In numerous microalgal species various lipid constituents are well documented with their percentage composition ranging from 20% up to 50% of dry biomass (w/w). It is a fact that limited nitrogen condition followed by intracellular lipid content rises considerably. Stationary phase cultures have also been reported with twice the amount of their neutral lipid constitution and produce more polysaccharides against proteins [24].
Figure 2: Omega-3 fatty acid.
Minerals and vitamins
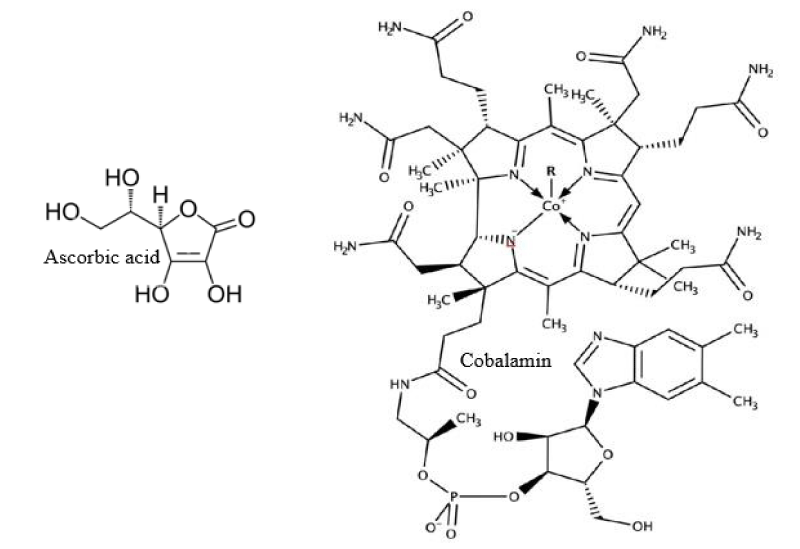
Seaweeds and microalgae are represented as plentiful sources of vitamins B1, B2, and cobalamin (Figure 3). A percentage of cobalamin as vitamin B12 was abundantly found in Rhodophyceae and Chlorophyceae as compared to phaeophycea [25]. Enteromorpha sp. and spirulina from green algae, and Porphyra sp., from red seaweed were found to have higher concentrations of vitamin B12. Almost all brown, red, and green seaweeds contain vitamin C (ascorbic acid). It plays an important role in anti-aging, immune stimulant activity, and radical scavenging activity which are some of the health advantages. Chondrus crispus, Gracilariopsis from Rhodophyta and Sargassum, and U. pinnatifida from Phaeophyceae are regarded as dietary supplements that fulfill daily intake recommendations of most minerals, like Na, Ca, K, and Mg. They are also found to have important trace elements; for example Zn, Fe, Mn, and Cu. Seaweeds are plentiful reservoirs of calcium. Including these in our diet will lower the risk of Ca deficiency in pregnant women [26].
Figure 3: Vitamins.
Highly valued natural product
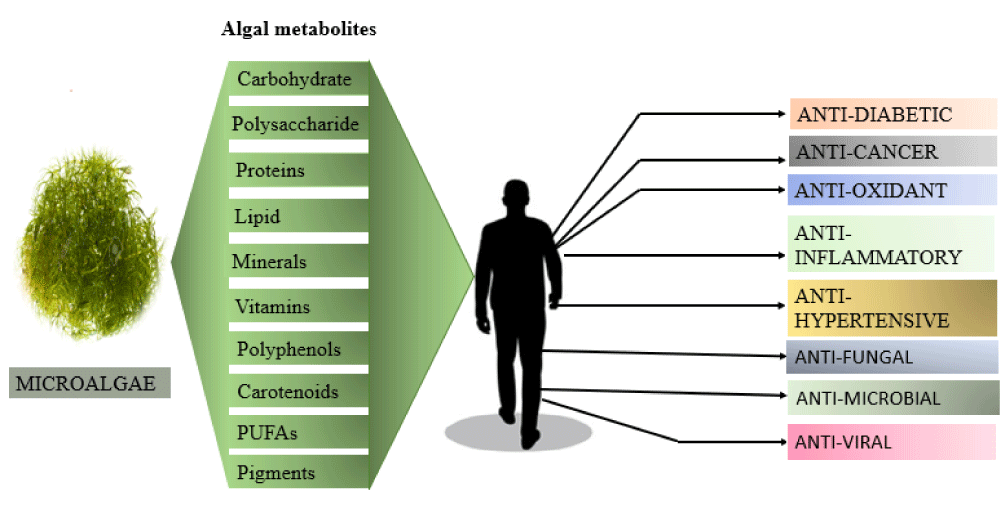
Due to a wide range of biological activities many natural microalgal products have received particular interest (Figure 4). Even though their final production volume is on a small scale, these types of products, from an industrial standpoint, are far more valuable economically as compared to whole, dried microalgal biomass. Fatty acids and Pigments are more easily available microalgal products sold on the market [27]. A green algae Dunaliella salina thrives in open ponds with higher salinity and light conditions used to produce a pigment called β-carotene. It was reported in 2010 that over 1200 tons of β-carotene were produced [28]. Another freshwater green alga, Haematococcus pluvialis found to produce a pigment called Astaxanthin. It is also a commercially important carotenoid. Comparatively, the production scale of astaxanthin is lower than β-carotene, still it’s market worth is still higher [29].
Figure 4: Depiction of Algal metabolites with their Pharmacological properties.
Pharmaceutical aspects
Antioxidant property: Growing concern over oxidative stress-related disorders leads to the generation of numerous curative strategies and indigenous remedies to address foods that are abundant in natural antioxidants. Since the discovery of novel metabolites with greater bioactivities than those found in terrestrial plants, attention to marine resources has gradually increased. It has been suggested that marine microalgae are interesting and significant producers of antioxidants [30,31]. This is probably related to their capability to endure a highly oxidizing environment, which elevates the concentration of antioxidants inside cells or stimulates the formation of antioxidant secondary metabolites. Carotenoids are a vital component in the process of photosynthesis. The primary role of these pigments is to capture light energy. On the other side, they are also acting as a photoprotective agent against free radicals and extreme environmental factors like UV and intense solar irradiation. Structural analysis of carotenoids is represented by the presence of conjugated double bonds or particular functional groups e.g., epoxy, allene (C=C=C), acetyl, acetylene groups (C≡C) indicating their antioxidation property [32]. It helps in the removal of singlet oxygen and free radicals from reactive species to neutralize them into harmless compounds. An in vitro study demonstrated that fucoxanthin, a brown pigment xanthophyll isolated from golden algae has shown 13 times more antioxidation properties than vitamin E, α-tocopherol. Phenolics have a broad range of physiochemical action including antioxidant activity. They typically provide defense against pathogens and protect from ultraviolet radiation. The chemical structure of phenolic compounds has a significant impact on their antioxidant action. There are some specific enzymes responsible for the production of ROS (reactive oxygen species) agents such as lipoxygenases, xanthin oxidase, and cyclooxygenase. Synergistic work of nonpolar benzenoid rings and hydroxyl groups of phenol and their derivatives ensure prevention of these enzymes leading to blockage of ROS production. The broad range of applications of bioactive peptides used for possible treatments of different pathological conditions as inflammation, diabetes, hypertension, and oxidative stress, has increased their demand on a global scale. The in vivo evaluation of lipid, protein peroxidation and DNA damage are the major checkpoints for detecting modification in anti-oxidant activity [33]. Enzymatic hydrolysis is a widely used method for the development of antioxidant peptides which have been evaluated as either crude hydrolysates or purified peptides. Navicula incerta undergoes papain enzyme breakdown giving rise to two bioactive peptides. The amino acid sequences of these two different peptides are Pro-Gly-Trp-Asn-Gln-Trp-Phe-Leu and Val-Glu-Val-Leu-Pro-Pro-Ala-Glu-Leu possess antioxidant action in HepG2/CYP2E1 cell line [34]. QSAR (Quantitative Structure-Activity Relationship) modeling of antioxidative peptides provides extra information about the more significant role of the C-terminal end over the N-terminal for scavenging radicals shows antioxidant potency.
Antihypertensive properties: Hypertension is a serious health problem at the global level. The need to control hypertension gives rise to ACE inhibitors. Bioactive peptides obtained from food are proven to have antihypertensive properties through the prevention of two enzymes i.e., ACE and renin responsible for the regulation of mammalian blood pressure. To date, certain no. of microalgae- Chlorella vulgaris, C. ellipsoidea, Nannochloropsis osculata, and Arthrospira platensis are being used to synthesize ACE-inhibiting peptides. A protein waste of algae, Chlorella vulgaris undergoes pepsin protease hydrolysis. This was followed by the purification of a peptide with a sequence of Val-Glu-Cys-Tyr-Gly-Pro-Asn-Arg-Pro-Gln-Phe performed significant inhibition of ACE enzyme with an IC50 value detected as 29.6 µM [35]. Another marine species of the Chlorella genus (Chlorella ellipsoidea) produces an ACE inhibitory oligopeptide consisting of four amino sequences, Val-Glu-Gly-Tyr possesses inhibition activity of IC50= 128.4 µM. Oral dosages of this oligopeptide exhibit an antihypertensive effect upon SHR (Spontaneously hypertensive rats). The main target of these ACE-inhibiting peptides is binding to the N-ter region or C-ter region of ACE active sites. Prior studies suggested that the presence of cyclic either aromatic amino acids (e.g., Pro, Phe, Tyr, Trp) at the C-terminal of peptide and aliphatic chains bearing nonpolar amino acids (e.g., Gly, Ile, Leu, Val) at their N-terminal end were supposed to promote ACE prevention [36].
Anti-inflammatory properties: Inflammation is a mechanism of innate immune defense in response to pathogens. It is a major contributor to type-2 diabetes, obesity, and finally cancer. ROS produced in brain tissue interferes with communication between synaptic and non-synaptic signaling in neurons leading to neuroinflammation and cellular death followed by neurodegenerative disease and dementia [37]. Microalgae natural products have been examined for their anti-inflammatory potential. Out of which carotenoids, modified carbohydrates, and PUFAs are more exploited compounds. Anti-inflammatory activities of algal extracts are generally attributed to inhibition of cytokines causing inflammation and eicosanoid production and downregulation of gene expression responsible for inflammation [38]. These algal metabolites exert their effects through different processes such as regulation of cellular reactions, inhibition of two important signal transduction pathways, and modulation of activities of enzymes- COX2 (cyclooxygenase-2), NOS (nitric oxide synthase), phospholipase A2. There is evidence that long-chain Polyunsaturated fatty acids involving Eicosapentaenoic acid and Docosahexaenoic acid have therapeutic effects against several inflammatory diseases like lupus, arthritis, and Alzheimer’s. According to Nauroth, et al. Schizochytrium sp. form an omega-3 PUFA, docosapentanoic acid. The use of DPA prevents lipopolysaccharide-triggered cytokine IL-1 β secretion and TNF-α in human peripheral blood mononuclear cells. According to Banskota, et al. [39], lipid extracts consisting of monogalactosyldiacylglycerols from Tetraselmis chuii, Chondrus crispus, and Chlorella sorokiniana performed anti-inflammation in RAW 264.7 macrophase cells. Using omega-3 fatty acids-rich microalgal oils as our diet has shown chemopreventive effects upon colonic aberrant crypt foci functions induced by azoxymethane. Decreased histamine secretion and modulating production of inflammatory cytokine generated an allergic reaction in human basophilic KU812F cells. Vo, et al. reported that PUFAs extracted from Ishige okamurae, brown seaweed mitigated inflammation generated by that allergic response [40]. Hence, micro or macro-algae-extracted lipid content abundant in PUFAs are supposed to be crucial edible ingredients to control inflammation. Targeting inflammation received considerable attention from Astaxanthin, a potent secondary metabolite. in vitro and in vivo studies demonstrated that microalgae, Haematococcus pluvialis produce Astaxanthin reduced inflammation induced by lipopolysaccharides [41]. Comparison between a common anti-inflammatory drug, Prednisolone and astaxanthin demonstrated that dosage conc. of 100mg/kg of astaxanthin had more potential than that standard drug conc. dose of 10mg/kg. It directly targets NO (nitrous oxide), and PGL2 (prostaglandin E2) production, reducing the concentration of TNF-α, IL-1β, and IL-6 proinflammatory cytokines. Mice infected with Helicobacter pylori received 10 10-day treatment exposure period of Astaxanthin doses (200 mg/kg) decreases the levels of gastric inflammation. This was generally attributed to blocking interferons that upregulate Interleukin-4 secretion in splenocytes followed by decreasing mucosa-related bacterial loads in the GI tract of infected mice. One of the best studied carbohydrates i.e., Sulfated polysaccharides isolated from both microalgae and macroalgae are inducing strong inflammatory action. Besides Porphyridium (Rodophyta), Phaeodactylum (Heterokonts), and Chlorella (Chlorophyta), there are several microalgae genus have been exploited for their anti-inflammatory activities. Reports of Guzman, et al. [42] demonstrated that crude extracts of polysaccharide from Chlorella stigmatophora and Phaeodactylum tricornutum show anti-inflammatory response in a paw edema test. According to Albuquerque, et al. the brown algae, Dictyota menstrualis formed a sulfated L-fucose, heterofucan to prevent inflammation. Heterofucan is bound to the transmembrane of polymorphonuclear cells and inhibits proinflammatory cytokine production. This leads to complete blockage of leucocyte transport into the peritoneal cavity in damaged tissue of mice. A fucoidan isolated from Laminaria japonica inactivates cytokines, and two immunoproteins (HMGB1 and NF-κB) secretion during inflammatory response and necrosis. It reduced inflammation of the injured myocardium layer in rats reported by Li, et al. [43].
Antidiabetic properties: Diabetes mellitus is a persistent metabolic condition followed by rising blood sugar levels resulting in cardiovascular diseases, renal dysfunction, and retinal damage. Maintaining the proper concentration of blood glucose and lipids through dietary management is a unique approach to treating diabetes [44]. Commercially accessible antidiabetic medications cause a number of unpleasant side effects throughout treatment. Considering this fact, researchers’ attention has recently focused on the discovery of antidiabetic drugs derived naturally with improved pharmacological efficacy and fewer side effects. By controlling different signaling pathways, natural bioactive compounds produced from microalgae showed antidiabetic properties, likewise, they carry out inhibition of enzymes like aldose reductase, α-glucosidase, α-amylase, dipeptidyl reductase and PTP 1B (protein tyrosine phosphatase 1B) enzyme [45]. In STZ-induced diabetic rats, fucoidan extracted from S. fusiforme reduced blood sugar levels, restored liver activity, and lowered oxidative stress [46]. Fucoidan extracted from Ecklonia maxima controlled type II diabetes by acting as a strong α-glucosidase inhibitor having a lower IC50 value (0.27-0.31 mg/ml) [47]. Additionally, in a rat model infected with type II diabetes (db/db) compilation of low molecular weight fucoidan (LMWF) extracted from S. hemiphyllum with fucoxanthin demonstrated antidiabetic potency. The decrease in urinal sugar level in the in vivo model represented that the combination drug effect was more effective. Lipid metabolism lets LMWF raise hepatic glycogen levels and antioxidative enzymes. The use of LMWF along with fucoxanthin regulated lipid metabolism through insulin receptor substrate (IRS-1), uncoupling protein (UCP)-1, GLUT (glucose transporter), and PPARϒ (peroxisome proliferator-activated receptor-gamma) level [48]. Further in a mouse model infected with diabetes(C57BL/KSJ/db/db), sulfated fucoidan extracted from Undaria pinnatifida prevented hyperglycemia by increasing insulin sensitivity. Sargassum wightii extracted fucoidan decreases blood glucose levels by preventing an enzyme α-D-glucosidase responsible for the transportation of glucose into the blood. Dieckol, 6,60-Bieckol, 7-phloroeckol, fucodiphloroethol G, phlorofucofuroeckol A, eckol from Ecklonia stolonifera & Eisenia bicyclis demonstrated strong α-glucosidase action and downregulate blood glucose level. In Sprague-Dawley diabetic rats, Pelvetia siliquosa extracted fucosterol decrease serum glucose conc. and prevent deposition of sorbitol in lens cells [49].
Future prospects
Microalgae may offer trustworthy and sustainable alternatives to frequently used commodities derived from plant or animal reservoirs with respect to expanding population and accessibility to terrestrial food sources. It will need a lot of studies and development to take algal products from a niche level to a hierarchy of being used widely as food commodities. This would involve genetic engineering-based enhancement of existing strains or scrutinizing novel species and modifications aimed at generating microalgae and increased production of specific metabolic compounds. After all, it is imperative that processing and scaling up microalgae should be carried out profitably. The target is to enable low-cost production in comparison to synthetic/ biological manufacture from other organisms. Despite the paucity of research on peptides belonging to microalgal origin, they have shown promising in vitro biological applications. Several methods, including chemical modification, microencapsulation, and nanoencapsulation are employed to restore the biological function of peptides. The tools of Bioinformatics improved understanding of the structure-function relationship enabling to production of peptides with increased bioactivity and to identification of undesirable side effects like hemolysis.
Currently, total microalgal biomass and isolated high-quality by-products are employed as components of nutraceuticals and functional foods. It is generally acknowledged that the biological and economic value of chemicals from microalgal extracts is higher than that of dried biomass. The total expenses of production including massive cultivation and regulation, low culture yield, and biorefining procedures, pose a challenge to delivering higher-quality algal products. Even though open ponds are the most cost-effective option for massive cultivation, one disadvantage is their susceptibility to contamination. That’s why closed PBR are considered over open ponds. Their purpose is to develop valuable products from selected strains that can meet the cost of the system. Emerging eco-friendly technologies including supercritical fluid extraction and enzymatic lysis are developed for the separation of higher-value algal substances. There is frequent demand for microalgal composition in foods, diet supplements, and pharma industries instead of the difficulties of cultivation in bulk amounts. Besides, much more study is required in this area to visualize the molecular composition of potential microalgae in order to completely evaluate their advantages and possible concerns. Although only a small number of production strains of microalgae have been studied out of thousands of anticipated strains, there are countless opportunities for uncovering a number of novel natural bioactive compounds. These metabolites should have multiple pharmacological properties i.e., antifungal, antimicrobial, and antitumorigenic related activities. Taking these properties into consideration, including these natural compounds in our diet fulfills our nutrition which is beneficial to human beings.
I would like to thank Dr. Amit Kumar, Department of Microbiology, Central University of Tamil Nadu, Thiruvarur, India for the support.
Authors’ contributions
Conception and design: PG; Development of methodology: PG, AB, ND, CB; Analysis and interpretation of data: PG; Revision of the manuscript: PG, Study supervision: PG, all authors read and approved the final manuscript.
Availability of data and materials: The data sets used and/or analysed during this study are available from the corresponding author upon reasonable request.
Consent for publication: All authors have agreed to publish this manuscript.
- McFadden GI. Primary and secondary endosymbiosis and the origin of plastids. J Phycol. 2001; 37(6):951-959. doi:10.1046/j.1529-8817.2001.01126.x
- Wright SW, Jeffrey SW. Pigment markers for phytoplankton production. Handb Environ Chem. 2006; (2):71-104. doi:10.1007/698_2_003
- Manning SR, Nobles DR. Impact of global warming on water toxicity: cyanotoxins. University of Texas at Austin, UTEX Culture Collection of Algae, Department of Molecular Biosciences; 2017:1-15.
- Demirbas A, Demirbas MF. Importance of algae oil as a source of biodiesel. Energy Convers Manage. 2011; 52(1):163-170. doi:10.1016/j.enconman.2010.06.055
- Hu GP, Yuan J, Sun L, She ZG, Wu JH, Lan XJ, Zhu X, Lin YC, Chen SP. Statistical research on marine natural products based on data obtained between 1985 and 2008. Mar Drugs. 2011;9(4):514-525. doi: 10.3390/md9040514. Epub 2011 Mar 29. PMID: 21731546; PMCID: PMC3124969.
- Goiris K, Muylaert K, Fraeye I, Foubert I, De Brabanter J, De Cooman L. Antioxidant potential of microalgae in relation to their phenolic and carotenoid content. J Appl Phycol. 2012; 24(6):1477-1486. doi:10.1007/s10811-012-9804-6
- Lauritano C, Andersen JH, Hansen E, Albrigtsen M, Escalera L, Esposito F, Helland K, Hanssen K, Romano G, Ianora A. Bioactivity screening of microalgae for antioxidant, anti-inflammatory, anticancer, anti-diabetes, and antibacterial activities. Front Mar Sci. 2016; 3:68. doi:10.3389/fmars.2016.00068
- Beetul K, Gopeechund A, Kaullysing D, Mattan-Moorgawa S, Puchooa D, Bhagooli R. Challenges and Opportunities in the Present Era of Marine Algal Applications. Algae - Organisms for Imminent Biotechnology. Published online 2016. doi:10.5772/63272
- Khan MI, Shin JH, Kim JD. The promising future of microalgae: current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb Cell Fact. 2018 Mar 5;17(1):36. doi: 10.1186/s12934-018-0879-x. PMID: 29506528; PMCID: PMC5836383.
- Koyande AK, Chew KW, Rambabu K, Tao Y, Chu DT, Show PL. Microalgae: A potential alternative to health supplementation for humans. Food Sci Hum Wellness. 2019; 8(1):16-24.
- Guedes AC, Amaro HM, Malcata FX. Microalgae as sources of carotenoids. Mar Drugs. 2011;9(4):625-644. doi: 10.3390/md9040625. Epub 2011 Apr 20. PMID: 21731554; PMCID: PMC3124977.
- Shaima AF, Mohd Yasin NH, Ibrahim N, Takriff MS, Gunasekaran D, Ismaeel MYY. Unveiling antimicrobial activity of microalgae Chlorella sorokiniana (UKM2), Chlorella sp. (UKM8) and Scenedesmus sp. (UKM9). Saudi J Biol Sci. 2022 Feb;29(2):1043-1052. doi: 10.1016/j.sjbs.2021.09.069. Epub 2021 Oct 2. PMID: 35197773; PMCID: PMC8848016.
- Bozarth A, Maier UG, Zauner S. Diatoms in biotechnology: modern tools and applications. Appl Microbiol Biotechnol. 2009 Feb;82(2):195-201. doi: 10.1007/s00253-008-1804-8. Epub 2008 Dec 11. PMID: 19082585.
- Lavrentyev PJ, Franzè G, Pierson JJ, Stoecker DK. The effect of dissolved polyunsaturated aldehydes on microzooplankton growth rates in the Chesapeake Bay and Atlantic coastal waters. Mar Drugs. 2015 May 6;13(5):2834-56. doi: 10.3390/md13052834. PMID: 25955757; PMCID: PMC4446608.
- Gastineau R, Turcotte F, Pouvreau JB, Morançais M, Fleurence J, Windarto E, Prasetiya FS, Arsad S, Jaouen P, Babin M, Coiffard L, Couteau C, Bardeau JF, Jacquette B, Leignel V, Hardivillier Y, Marcotte I, Bourgougnon N, Tremblay R, Mouget JL. Marennine, promising blue pigments from a widespread Haslea diatom species complex. Mar Drugs. 2014; 12(6):3161-3189. doi:10.3390/md12063161
- Zaid AAA, Hammad DM, Sharaf EM. Antioxidant and anticancer activity of spirulina platensis water extracts. Int J Pharmacol. 2015; 11(7):846-851. doi:10.3923/ijp.2015.846.851
- Yim HE, Yoo KH, Seo WH, Won NH, Hong YS, Lee JW. Acute tubulointerstitial nephritis following ingestion of Chlorella tablets. Pediatr Nephrol. 2007 Jun;22(6):887-8. doi: 10.1007/s00467-006-0420-z. Epub 2007 Feb 2. PMID: 17273860.
- Dietrich D, Agency GF, Assessment R, Chlorella W, Lake K, Dietrich D. Food supplements from blue-green algae do more harm than good. Gesundheitsindustrie BW. Accessed June 17, 2024. https://www.gesundheitsindustrie-bw.de/en/article/news/food-supplements-from-blue-green-algae-do-more-harm-than-good/
- Plaza M, Herrero M, Cifuentes A, Ibáñez E. Innovative natural functional ingredients from microalgae. J Agric Food Chem. 2009 Aug 26;57(16):7159-70. doi: 10.1021/jf901070g. PMID: 19650628.
- Nakamura Y, Takahashi JI, Sakurai A, Inaba Y, Suzuki E, Nihei S, Fujiwara S, Tsuzuki M, Miyashita H, Ikemoto H, Kawachi M, Sekiguchi H, Kurano N. Some cyanobacteria synthesize semi-amylopectin type α-polyglucans instead of glycogen. Plant Cell Physiol. 2005; 46(3):539-545. doi:10.1093/pcp/pci045
- Busi MV, Barchiesi J, Martín M, Gomez-Casati DF. Starch metabolism in green algae. Starch/Staerke. 2014; 66(1-2):28-40. doi:10.1002/star.201200211
- Cain DJR. AMYLOSE in floridean starch. 1981:67-71.
- Qu L, Ren LJ, Huang H. Scale-up of docosahexaenoic acid production in fed-batch fermentation by Schizochytrium sp. based on volumetric oxygen-transfer coefficient. Biochem Eng J. 2013; 77:82-87. doi:10.1016/j.bej.2013.05.011
- Muller-Feuga A, Moal J, Kaas R. The Microalgae of Aquaculture. In: Live Feeds in Marine Aquaculture. November 2007. doi:10.1002/9780470995143.ch6
- Circuncisão AR, Catarino MD, Cardoso SM, Silva AMS. Minerals from Macroalgae Origin: Health Benefits and Risks for Consumers. Mar Drugs. 2018 Oct 23;16(11):400. doi: 10.3390/md16110400. PMID: 30360515; PMCID: PMC6266857.
- Rupérez P. Mineral content of edible marine seaweeds. Food Chem. 2002; 79(1):23-26. doi:10.1016/S0308-8146(02)00171-1
- Minhas AK, Hodgson P, Barrow CJ, Adholeya A. A Review on the Assessment of Stress Conditions for Simultaneous Production of Microalgal Lipids and Carotenoids. Front Microbiol. 2016 May 3;7:546. doi: 10.3389/fmicb.2016.00546. PMID: 27199903; PMCID: PMC4853371.
- Coesel SN, Baumgartner AC, Teles LM, Ramos AA, Henriques NM, Cancela L, Varela JC. Nutrient limitation is the main regulatory factor for carotenoid accumulation and for Psy and Pds steady state transcript levels in Dunaliella salina (Chlorophyta) exposed to high light and salt stress. Mar Biotechnol (NY). 2008 Sep-Oct;10(5):602-11. doi: 10.1007/s10126-008-9100-2. Epub 2008 May 1. PMID: 18449600.
- Panis G, Carreon JR. Commercial astaxanthin production derived by green alga Haematococcus pluvialis: A microalgae process model and a techno-economic assessment all through production line. Algal Res. 2016; 18:175-190. doi:10.1016/j.algal.2016.06.007
- Xia S, Gao B, Li A, Xiong J, Ao Z, Zhang C. Preliminary characterization, antioxidant properties and production of chrysolaminarin from marine diatom Odontella aurita. Mar Drugs. 2014 Sep 23;12(9):4883-97. doi: 10.3390/md12094883. PMID: 25251034; PMCID: PMC4178495.
- Xia S, Wang K, Wan L, Li A, Hu Q, Zhang C. Production, characterization, and antioxidant activity of fucoxanthin from the marine diatom Odontella aurita. Mar Drugs. 2013 Jul 23;11(7):2667-81. doi: 10.3390/md11072667. PMID: 23880936; PMCID: PMC3736445.
- Raposo MF, de Morais AM, de Morais RM. Carotenoids from Marine Microalgae: A Valuable Natural Source for the Prevention of Chronic Diseases. Mar Drugs. 2015 Aug 14;13(8):5128-55. doi: 10.3390/md13085128. PMID: 26287216; PMCID: PMC4557017.
- Alam MN, Bristi NJ, Rafiquzzaman M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm J. 2013 Apr;21(2):143-52. doi: 10.1016/j.jsps.2012.05.002. Epub 2012 Jun 15. PMID: 24936134; PMCID: PMC4052538.
- Kang KH, Qian ZJ, Ryu B, Karadeniz F, Kim D, Kim SK. Antioxidant peptides from protein hydrolysate of microalgae Navicula incerta and their protective effects in HepG2/CYP2E1 cells induced by ethanol. Phytother Res. 2012 Oct;26(10):1555-63. doi: 10.1002/ptr.4603. Epub 2012 Mar 19. PMID: 22431441.
- Sheih IC, Fang TJ, Wu TK. Isolation and characterisation of a novel angiotensin I-converting enzyme (ACE) inhibitory peptide from the algae protein waste. Food Chem. 2009; 115(1):279-284. doi:10.1016/j.foodchem.2008.12.019
- Lancaster JR Jr. Sickle cell disease: loss of the blood's WD40? Trends Pharmacol Sci. 2003 Aug;24(8):389-91. doi: 10.1016/S0165-6147(03)00199-8. PMID: 12915046.
- Popa-Wagner A, Mitran S, Sivanesan S, Chang E, Buga AM. ROS and brain diseases: the good, the bad, and the ugly. Oxid Med Cell Longev. 2013;2013:963520. doi: 10.1155/2013/963520. Epub 2013 Dec 5. PMID: 24381719; PMCID: PMC3871919.
- Robertson RC, Guihéneuf F, Bahar B, Schmid M, Stengel DB, Fitzgerald GF, Ross RP, Stanton C. The Anti-Inflammatory Effect of Algae-Derived Lipid Extracts on Lipopolysaccharide (LPS)-Stimulated Human THP-1 Macrophages. Mar Drugs. 2015 Aug 20;13(8):5402-24. doi: 10.3390/md13085402. PMID: 26308008; PMCID: PMC4557028.
- Banskota AH, Gallant P, Stefanova R, Melanson R, O'Leary SJ. Monogalactosyldiacylglycerols, potent nitric oxide inhibitors from the marine microalga Tetraselmis chui. Nat Prod Res. 2013;27(12):1084-90. doi: 10.1080/14786419.2012.717285. Epub 2012 Sep 14. PMID: 22973805.
- Vo TS, Kim JA, Wijesekara I, Kong CS, Kim SK. Potent effect of brown algae (Ishige okamurae) on suppression of allergic inflammation in human basophilic KU812F cells. Food Sci Biotechnol. 2011; 20(5):1227-1234. doi:10.1007/s10068-011-0169-4
- Ohgami K, Shiratori K, Kotake S, Nishida T, Mizuki N, Yazawa K, Ohno S. Effects of astaxanthin on lipopolysaccharide-induced inflammation in vitro and in vivo. Invest Ophthalmol Vis Sci. 2003 Jun;44(6):2694-701. doi: 10.1167/iovs.02-0822. PMID: 12766075.
- Guzmán S, Gato A, Lamela M, Freire-Garabal M, Calleja JM. Anti-inflammatory and immunomodulatory activities of polysaccharide from Chlorella stigmatophora and Phaeodactylum tricornutum. Phytother Res. 2003 Jun;17(6):665-70. doi: 10.1002/ptr.1227. PMID: 12820237.
- Lee H, Kim JS, Kim E. Fucoidan from seaweed Fucus vesiculosus inhibits migration and invasion of human lung cancer cell via PI3K-Akt-mTOR pathways. PLoS One. 2012;7(11):e50624. doi: 10.1371/journal.pone.0050624. Epub 2012 Nov 30. PMID: 23226337; PMCID: PMC3511566.
- Doyle A. Microalbuminuria in diabetic patients. Nurs Times. 1991; 87(28):43.
- Gupta P, Bala M, Gupta S, Dua A, Dabur R, Injeti E, Mittal A. Efficacy and risk profile of anti-diabetic therapies: Conventional vs traditional drugs-A mechanistic revisit to understand their mode of action. Pharmacol Res. 2016 Nov;113(Pt A):636-674. doi: 10.1016/j.phrs.2016.09.029. Epub 2016 Sep 30. PMID: 27697646.
- Cheng Y, Sibusiso L, Hou L, Jiang H, Chen P, Zhang X, Wu M, Tong H. Sargassum fusiforme fucoidan modifies the gut microbiota during alleviation of streptozotocin-induced hyperglycemia in mice. Int J Biol Macromol. 2019 Jun 15;131:1162-1170. doi: 10.1016/j.ijbiomac.2019.04.040. Epub 2019 Apr 8. PMID: 30974142.
- Daub CD, Mabate B, Malgas S, Pletschke BI. Fucoidan from Ecklonia maxima is a powerful inhibitor of the diabetes-related enzyme, α-glucosidase. Int J Biol Macromol. 2020 May 15;151:412-420. doi: 10.1016/j.ijbiomac.2020.02.161. Epub 2020 Feb 16. PMID: 32070744.
- Kim KT, Rioux LE, Turgeon SL. Alpha-amylase and alpha-glucosidase inhibition is differentially modulated by fucoidan obtained from Fucus vesiculosus and Ascophyllum nodosum. Phytochemistry. 2014 Feb;98:27-33. doi: 10.1016/j.phytochem.2013.12.003. Epub 2013 Dec 30. PMID: 24388677.
- Lee YS, Shin KH, Kim BK, Lee S. Anti-diabetic activities of fucosterol from Pelvetia siliquosa. Arch Pharm Res. 2004 Nov;27(11):1120-2. doi: 10.1007/BF02975115. PMID: 15595413.